Chem Catalysis ( IF 11.5 ) Pub Date : 2022-04-12 , DOI: 10.1016/j.checat.2022.03.005 Luca Capaldo 1 , Timothy Noël 1 , Davide Ravelli 2
|
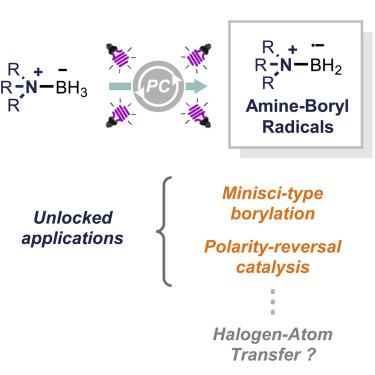
Photocatalysis has recently given impetus to the use of ligated boryl radicals (LBRs) in synthesis, thanks to the mild conditions required for their generation based on the use of visible light. LBRs are B-centered radicals wherein the boron atom is coordinated with a suitable Lewis base (e.g., amine, phosphine, or N-heterocyclic carbene [NHC]) and can be conveniently accessed from the corresponding ligated boranes through the cleavage of a B−H bond. While NHC-boranes featuring a rather labile B−H bond are routinely used in photocatalytic strategies, this perspective highlights the recent adoption of more challenging tertiary amine-boranes, which unlocked unprecedented reaction manifolds. The highlighted applications include the Minisci-type borylation of azines and the implementation of polarity-reversal catalysis for the generation of electrophilic C-centered radicals via hydrogen-atom transfer (HAT). The possibility to devise an analogous strategy based on halogen-atom transfer (XAT) is also discussed.
中文翻译:

叔胺-硼烷配合物光催化生成配位硼自由基:有机合成中的新兴工具
由于基于可见光的使用产生它们所需的温和条件,光催化最近推动了在合成中使用连接的硼自由基 (LBR)。LBR 是 B 中心基团,其中硼原子与合适的路易斯碱(例如胺、膦或N-杂环卡宾 [NHC]),并且可以通过 B-H 键的断裂从相应的连接硼烷方便地获得。虽然具有相当不稳定的 B-H 键的 NHC-硼烷通常用于光催化策略,但这一观点强调了最近采用更具挑战性的叔胺-硼烷,它解锁了前所未有的反应歧管。突出的应用包括吖嗪的 Minisci 型硼基化和通过氢原子转移 (HAT) 产生亲电 C 中心自由基的极性反转催化。还讨论了设计基于卤素原子转移 (XAT) 的类似策略的可能性。































 京公网安备 11010802027423号
京公网安备 11010802027423号