Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2022-04-11 , DOI: 10.1016/j.bmc.2022.116743 Meena V Patel 1 , Hillary M Peltier 1 , Mark A Matulenko 1 , John R Koenig 1 , Marc J C Scanio 1 , Rebecca J Gum 1 , Odile F El-Kouhen 1 , Meagan M Fricano 1 , Greta L Lundgaard 1 , Torben Neelands 1 , Xu-Feng Zhang 1 , Cenchen Zhan 1 , Madhavi Pai 1 , Nayereh Ghoreishi-Haack 1 , Thomas Hudzik 1 , Gary Gintant 1 , Ruth Martin 1 , Steve McGaraughty 1 , Jun Xu 1 , Daniel Bow 1 , John C Kalvass 1 , Philip R Kym 1 , David A DeGoey 1 , Michael E Kort 1
|
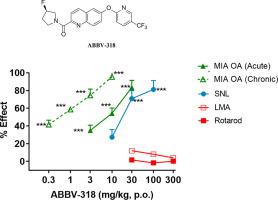
The voltage-gated sodium channel Nav1.7 is an attractive target for the treatment of pain based on the high level of target validation with genetic evidence linking Nav1.7 to pain in humans. Our effort to identify selective, CNS-penetrant Nav1.7 blockers with oral activity, improved selectivity, good drug-like properties, and safety led to the discovery of 2-substituted quinolines and quinolones as potent small molecule Nav1.7 blockers. The design of these molecules focused on maintaining potency at Nav1.7, improving selectivity over the hERG channel, and overcoming phospholipidosis observed with the initial leads. The structure-activity relationship (SAR) studies leading to the discovery of (R)-(3-fluoropyrrolidin-1-yl)(6-((5-(trifluoromethyl)pyridin-2-yl)oxy)quinolin-2-yl)methanone (ABBV-318) are described herein. ABBV-318 displayed robust in vivo efficacy in both inflammatory and neuropathic rodent models of pain. ABBV-318 also inhibited Nav1.8, another sodium channel isoform that is an active target for the development of new pain treatments.
中文翻译:

(R)-(3-fluoropyrrolidin-1-yl)(6-((5-(trifluoromethyl)pyridin-2-yl)oxy)quinolin-2-yl)methanone (ABBV-318) 及其类似物小分子的发现Nav1.7/ Nav1.8 阻滞剂治疗疼痛
电压门控钠通道 Na v 1.7 是治疗疼痛的一个有吸引力的目标,基于高水平的目标验证以及将 Na v 1.7 与人类疼痛联系起来的遗传证据。我们努力识别具有口服活性、选择性提高、良好的药物样特性和安全性的选择性、CNS 渗透性 Na v 1.7 阻滞剂,导致发现 2-取代的喹啉和喹诺酮类药物是有效的小分子 Na v 1.7 阻滞剂。这些分子的设计侧重于保持 Na v 1.7 的效力,提高对 hERG 通道的选择性,并克服在初始引线中观察到的磷脂沉积。结构-活性关系 (SAR) 研究导致发现 ( R)-(3-氟吡咯烷-1-基)(6-((5-(三氟甲基)吡啶-2-基)氧基)喹啉-2-基)甲酮(ABBV-318)在本文中进行了描述。ABBV-318在炎症性和神经性啮齿动物疼痛模型中显示出强大的体内功效。ABBV-318 还抑制 Na v 1.8,这是另一种钠通道异构体,是开发新的疼痛治疗的积极目标。






























 京公网安备 11010802027423号
京公网安备 11010802027423号