European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2022-04-11 , DOI: 10.1016/j.ejmech.2022.114342 Petja Rosenqvist 1 , Janne J Mäkinen 2 , Kaisa Palmu 2 , Johanna Jokinen 2 , Ranjit K Prajapati 2 , Heidi J Korhonen 1 , Pasi Virta 1 , Georgiy A Belogurov 2 , Mikko Metsä-Ketelä 2
|
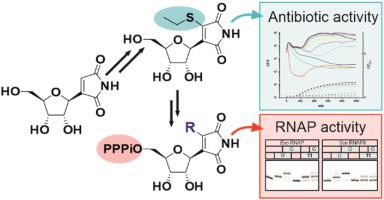
Showdomycin produced by Streptomyces showdoensis ATCC 15227 is a C-nucleoside microbial natural product with antimicrobial and cytotoxic properties. The unique feature of showdomycin in comparison to other nucleosides is its maleimide base moiety, which has the distinct ability to alkylate nucleophilic thiol groups by a Michael addition reaction. In order to understand structure-activity relationships of showdomycin, we synthesized a series of derivatives with modifications in the maleimide ring at the site of alkylation to moderate its reactivity. The showdomycin congeners were designed to retain the planarity of the base ring system to allow Watson–Crick base pairing and preserve the nucleosidic character of the compounds. Consequently, we synthesized triphosphates of showdomycin derivatives and tested their activity against RNA polymerases. Bromo, methylthio, and ethylthio derivatives of showdomycin were incorporated into RNA by bacterial and mitochondrial RNA polymerases and somewhat less efficiently by the eukaryotic RNA polymerase II. Showdomycin derivatives acted as uridine mimics and delayed further extension of the RNA chain by multi-subunit, but not mitochondrial RNA polymerases. Bioactivity profiling indicated that the mechanism of action of ethylthioshowdomycin was altered, with approximately 4-fold reduction in both cytotoxicity against human embryonic kidney cells and antibacterial activity against Escherichia coli. In addition, the ethylthio derivative was not inactivated by medium components or influenced by addition of uridine in contrast to showdomycin. The results explain how both the maleimide ring and the nucleoside nature contribute to the bioactivity of showdomycin and demonstrates for the first time that the two activities can be separated.
中文翻译:

马来酰亚胺环系对秀多霉素构效关系的作用
由Streptomyces showdoensis 生产的 ShowdomycinATCC 15227 是一种 C-核苷微生物天然产物,具有抗菌和细胞毒性特性。与其他核苷相比,秀多霉素的独特之处在于其马来酰亚胺碱基部分,它具有通过迈克尔加成反应将亲核硫醇基团烷基化的独特能力。为了了解showdomycin的构效关系,我们合成了一系列衍生物,在烷基化位点的马来酰亚胺环上进行了修饰,以调节其反应性。展示霉素同系物旨在保留碱基环系统的平面性,以允许 Watson-Crick 碱基配对并保留化合物的核苷特性。因此,我们合成了秀多霉素衍生物的三磷酸盐,并测试了它们对 RNA 聚合酶的活性。溴、甲硫基、秀多霉素和乙硫基衍生物通过细菌和线粒体 RNA 聚合酶掺入 RNA,而通过真核 RNA 聚合酶 II 的效率稍低。Showdomycin 衍生物充当尿苷模拟物并通过多亚基延迟 RNA 链的进一步延伸,但不是线粒体 RNA 聚合酶。生物活性分析表明,乙基硫显示霉素的作用机制发生了改变,对人胚胎肾细胞的细胞毒性和对人胚胎肾细胞的抗菌活性均降低了约 4 倍。但不是线粒体 RNA 聚合酶。生物活性分析表明,乙基硫显示霉素的作用机制发生了改变,对人胚胎肾细胞的细胞毒性和对人胚胎肾细胞的抗菌活性均降低了约 4 倍。但不是线粒体 RNA 聚合酶。生物活性分析表明,乙基硫显示霉素的作用机制发生了改变,对人胚胎肾细胞的细胞毒性和对人胚胎肾细胞的抗菌活性均降低了约 4 倍。大肠杆菌。此外,与显示霉素相比,乙硫基衍生物不受培养基成分的灭活或尿苷添加的影响。结果解释了马来酰亚胺环和核苷性质如何促进秀多霉素的生物活性,并首次证明这两种活性可以分开。

































 京公网安备 11010802027423号
京公网安备 11010802027423号