Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2022-04-12 , DOI: 10.1016/j.bioorg.2022.105804 Junfeng Wang 1 , Kazue Takahashi 1 , Timothy M Shoup 1 , Lichong Gong 2 , Yingbo Li 3 , Georges El Fakhri 1 , Zhaoda Zhang 2 , Anna-Liisa Brownell 1
|
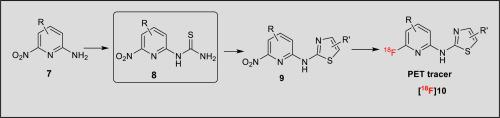
A novel organomediated cleavage of benzoyl group using ethane-1,2-diamine and acetic acid under neutral condition enables an efficient synthesis of 1-(6-nitropyridin-2-yl)thiourea, which previously has been challenging to prepare by conventional methods. The successful synthesis of 1-(6-nitropyridin-2-yl)thiourea as a synthon permits development of a variety of 18F labeled heterocycles as PET imaging ligands such as N-(pyridin-2-yl)thiazol-2-amine derivatives. The utility of this synthon is demonstrated with the synthesis of a 18F-labeled PET tracer for studying prion disease. In vitro autoradiography using this PET tracer on sagittal rat brain slices showed highest accumulation of radioactivity in the hippocampus, cortex, and striatum, in accordance with reported immunostaining of PrPc in rat brain.
中文翻译:

有机介导的苯甲酰基裂解能够有效合成 1-(6-nitropyridin-2-yl) 硫脲及其在开发 18F 标记的 PET 示踪剂中的应用
在中性条件下使用乙烷-1,2-二胺和乙酸进行的新型有机介导的苯甲酰基裂解能够有效合成 1-(6-nitropyridin-2-yl)thiourea,这在以前通过常规方法制备具有挑战性。1-(6-nitropyridin-2-yl)thiourea 作为合成子的成功合成允许开发各种18 F 标记的杂环作为 PET 成像配体,例如N- (pyridin-2-yl)thiazol-2-amine 衍生物. 通过合成18 F 标记的 PET 示踪剂来研究朊病毒病,证明了这种合成子的实用性。体外根据报告的大鼠脑中 PrP c的免疫染色,使用这种 PET 示踪剂对矢状大鼠脑切片进行放射自显影显示海马、皮层和纹状体中的放射性积累最高。































 京公网安备 11010802027423号
京公网安备 11010802027423号