Nano Research ( IF 9.5 ) Pub Date : 2022-04-08 , DOI: 10.1007/s12274-022-4245-2
Longxin Chen 1 , Ting Liu 1 , Duobin Chao 1
|
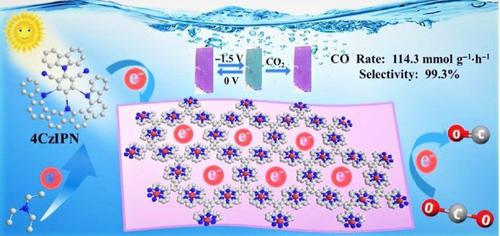
A coordination nanosheet composed of [Fe(tpy)2]2+ (tpy = 2,2′:6′,2″-terpyridine) units, showing reversible electrochromism at the ligand-based cathodic potential, has been prepared through a liquid/liquid interfacial synthesis. The noble metal-free nanosheet exhibited a CO evolution rate of 114.3 mmol·g−1·h−1 with the selectivity up to 99.3% under visible light irradiation in the presence of water, which is in the front rank of heterogeneous catalysis for CO2 photoreduction. Such robust photocatalytic performance is due to efficient ligand-based electron transfer through long-lived π radical anion tpy− with a lifetime more than 25 min, as evidenced by in situ electron paramagnetic resonance (EPR) and ultraviolet—visible—near infrared (UV—vis—NIR) spectroscopy studies. Fe(II) cation in [Fe(tpy)2]2+ mainly contributes to enhancing reduction potentials of ligand and stabilizing π radical anion tpy−. This ligand-based electron transfer with the aid of metal cation represents a promising strategy for selective CO2 photoreduction, especially towards gaining CO from CO2.
中文翻译:

一种电致变色配位纳米片,用于通过基于配体的电子转移实现稳健的 CO2 光还原
通过液体/ _ _液体界面合成。无贵金属纳米片在水存在下可见光照射下CO的析出率为114.3 mmol·g -1 ·h -1,选择性高达99.3%,处于CO非均相催化的前列2光还原。这种强大的光催化性能是由于有效的基于配体的电子通过长寿命的 π 自由基阴离子 tpy -进行的,寿命超过 25 分钟,原位证明电子顺磁共振 (EPR) 和紫外-可见-近红外 (UV-vis-NIR) 光谱研究。[Fe(tpy) 2 ] 2+中的Fe(II)阳离子主要有助于提高配体的还原电位和稳定π自由基阴离子tpy -。这种借助金属阳离子的基于配体的电子转移代表了一种有前途的选择性CO 2光还原策略,特别是从CO 2中获得CO 。







































 京公网安备 11010802027423号
京公网安备 11010802027423号