Chemical Engineering Science ( IF 4.1 ) Pub Date : 2022-04-09 , DOI: 10.1016/j.ces.2022.117657 Shenfang Li 1 , Xunli Zhang 2 , Desheng Ji 1 , Qingqiang Wang 1 , Nan Jin 1 , Yuchao Zhao 1
|
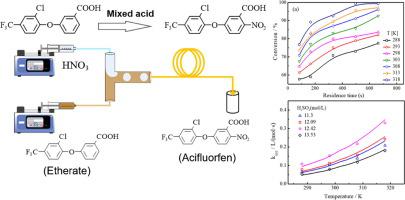
Aromatic nitration still faces unmet technical challenges in relation to being heterogeneous and highly exothermic. In this work, a continuous flow aromatic nitration was developed with mixed acid within droplet-based microreactors. The effects of key operating parameters were characterized on the nitration of 3-[2-chloro-4-(trifluoromethyl)phenoxy] benzoic acid. The optimized reaction temperature, M-ratio and N/S were found to be 308 K, 1.6 and 0.57, respectively. Under these conditions, a conversion of 83.03% and selectivity of 79.52% were achieved. The addition of acetic anhydride improved both the solubility of etherate in the organic phase and the absorbability of water produced from the reaction, leading to increase in conversion, however increased by-products accordingly with reduced selectivity. Pseudo-homogeneous kinetics models were developed of apparent and intrinsic reaction rate constant, supporting the second-order reaction assumption. The calculated results based on the kinetics model were found to be in good agreement with the experimental results.
中文翻译:

3-[2-氯-4-(三氟甲基)苯氧基]苯甲酸的连续流动硝化及其在液滴微反应器中的化学动力学
芳香硝化仍然面临着与多相和高度放热相关的未解决的技术挑战。在这项工作中,在基于液滴的微反应器中使用混合酸开发了一种连续流动的芳香族硝化。关键操作参数对3-[2-氯-4-(三氟甲基)苯氧基]苯甲酸硝化的影响进行了表征。发现优化的反应温度、M 比和 N/S 分别为 308 K、1.6 和 0.57。在这些条件下,实现了 83.03% 的转化率和 79.52% 的选择性。添加乙酸酐提高了醚合物在有机相中的溶解度和反应产生的水的吸收能力,导致转化率增加,但相应地增加了副产物,降低了选择性。建立了表观和内在反应速率常数的伪均相动力学模型,支持二级反应假设。基于动力学模型的计算结果与实验结果吻合较好。






























 京公网安备 11010802027423号
京公网安备 11010802027423号