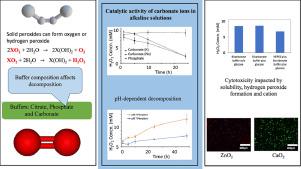
固体过氧化物提供持续释放氧气和过氧化氢的能力使其可能适用于氧气释放或抗菌应用。最近使用固体过氧化物来增加氧气含量的报告是通过在聚合物中混合固体过氧化物粉末来延缓水分解来实现的。可以添加具有过氧化物酶活性的化合物以降低过氧化氢毒性。过氧化物很少是纯的并且与氧化物混合并且它们本身在水中分解形成氢氧化物。因此,即使使用缓冲策略,水浸过氧化物颗粒表面的局部 pH 值也不可避免地呈碱性。由于 pH 影响过氧化物的分解和过氧化氢的稳定性,该研究首次比较了已在临床前评估的正在使用或已用于医疗应用的氢和无机过氧化物的水分解产物;过氧化钙(CaO2 )、过氧化镁(MgO 2 )、过氧化锌(ZnO 2 )、过碳酸钠(Na 2 CO 3 .1.5H 2 O 2 )和过氧化氢(H 2 O 2 )。由于血浆可以近似为碳酸盐缓冲的磷酸盐溶液,我们使用碳酸盐和磷酸盐缓冲液维持 pH 值,并将结果与柠檬酸盐缓冲液进行比较。对于给定的过氧化物化合物,我们不仅确定了 pH 值的强烈影响,而且还确定了缓冲液成分对氧和过氧化氢形成程度的影响。以前没有意识到缓冲液成分的影响,从而在体外建立用于更好地设计特定分解物质的有意释放的参数。
重要性声明
本文首次比较了金属阳离子(钙、镁、钠和锌)的固体过氧化物(钙、镁、钠和锌)在体内可能存在的 pH 值范围内(pH 5,7)的水分解产物氧和过氧化氢。 , 9) 无论是生理上还是病理上。我们发现,除了 pH 值外,缓冲液成分也是一个至关重要的因素,这使得体外模型的转化具有挑战性。细胞毒性与过氧化氢释放、碱度有关,在过氧化锌的情况下与阳离子本身有关。体外和临床前研究通常报告聚合物-过氧化物复合材料的释放数据,很少将过氧化物相互比较。我们的数据共同为这些无机材料的氧气和 ROS 输送提供了指导。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Aqueous decomposition behavior of solid peroxides: Effect of pH and buffer composition on oxygen and hydrogen peroxide formation
The ability of solid peroxides to provide sustained release of both oxygen and hydrogen peroxide makes them potentially suitable for oxygen release or antibacterial applications. Most recent reports using solid peroxides to augment oxygen levels do so by compounding solid peroxide powders in polymers to retard the aqueous decomposition. Compounds with peroxidase activity may be added to reduce hydrogen peroxide toxicity. Peroxides are rarely pure and are mixed with oxide and themselves decompose to form hydroxides in water. Therefore, even if buffering strategies are used, locally the pH at the surface of aqueously immersed peroxide particles is inevitably alkaline. Since pH affects the decomposition of peroxides and hydrogen peroxide stability, this study compared for the first-time the aqueous decomposition products of hydrogen and inorganic peroxides that are in use or have been used for medical applications of have been evaluated preclinically; calcium peroxide (CaO2), magnesium peroxide (MgO2), zinc peroxide (ZnO2), sodium percarbonate (Na2CO3.1.5H2O2) and hydrogen peroxide (H2O2). Since plasma can be approximated to be carbonate buffered phosphate solution, we maintained pH using carbonate and phosphate buffers and compared results with citrate buffers. For a given peroxide compound, we identified not only a strong effect of pH but also of buffer composition on the extent to which oxygen and hydrogen peroxide formation occurred. The influence of buffer composition was not previously appreciated, thereby establishing in vitro parameters for better design of intentional release of specific decomposition species.
Statement of significance
This paper compares for the first time the aqueous decomposition products oxygen and hydrogen peroxide of solid peroxy compounds of metal cations, (calcium, magnesium, sodium and zinc) across a pH range that could feasibly be found in the body, (pH 5,7, 9) either physiologically or pathologically. We find that in addition to pH, buffer composition is also a critically important factor, making translation from in vitro models challenging. Cytotoxicity was related to hydrogen peroxide release, alkalinity and in the case of zinc peroxide to the cation itself. In vitro and preclinical studies generally report release data from polymer-peroxide composites and rarely compare peroxides with one another. Together our data provide guidance for oxygen and ROS delivery from these inorganic materials.






















































 京公网安备 11010802027423号
京公网安备 11010802027423号