当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogenolysis Cleavage of the Csp2–Csp3 Bond over a Metal-Free NbOPO4 Catalyst
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-04-07 , DOI: 10.1021/acscatal.2c00034
Hao Zhou 1 , Lu Chen 2 , Yong Guo 1 , Xiaohui Liu 1 , Xin-Ping Wu 2 , Xue-Qing Gong 2 , Yanqin Wang 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-04-07 , DOI: 10.1021/acscatal.2c00034
Hao Zhou 1 , Lu Chen 2 , Yong Guo 1 , Xiaohui Liu 1 , Xin-Ping Wu 2 , Xue-Qing Gong 2 , Yanqin Wang 1
Affiliation
|
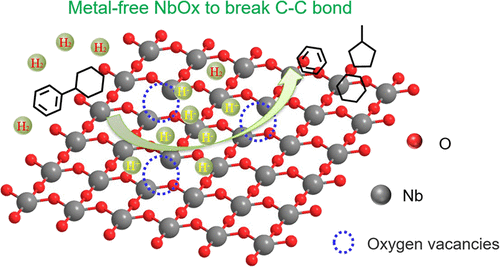
Ru/NbOx catalysts, which combine the merits of facile hydrogen activation, a strong binding ability to the benzene ring, and the presence of Brønsted acid sites, were well investigated toward Csp2–Csp3 bond cleavage in the utilization of lignin and aromatic plastics (Chem 2019, 5, 1521; Angew. Chem. Int. Ed. 2021, 60, 5527). Herein, we unlock the ability of a bare NbOx catalyst in the dissociation and activation of a hydrogen molecule and further hydrogenolysis of the robust Csp2–Csp3 model compounds. In situ diffuse reflectance infrared Fourier transform and density functional theory calculations reveal that H2 can be dissociated and the stable surface hydride species can be produced over Nb2O5 through heterolytic and homolytic cleavages of H2 due to the existence of surface oxygen vacancies. Furthermore, the NbOPO4 catalyst with rich oxygen vacancy and Brønsted acidity not only allows the conversion of phenylcyclohexane to monocyclic compounds but also enables the conversion of polystyrene to arenes with a high selectivity by cleaving the Csp2–Csp3 bond. This study provides and proves the unique ability of the NbOPO4 catalyst in heterolytic and homolytic cleavages of H2 and the hydrogenolysis of robust Csp2–Csp3 bond containing compounds, including plastics, which would help to design low-cost metal-free hydrogenolysis catalysts.
中文翻译:

Csp2-Csp3 键在无金属 NbOPO4 催化剂上的氢解裂解
Ru/NbO x催化剂结合了易于氢活化、与苯环的强结合能力以及存在布朗斯台德酸位点等优点,在利用木质素和芳香族化合物中对 C sp2 -C sp3键断裂进行了深入研究。塑料(Chem 2019, 5 , 1521;Angew. Chem. Int. Ed. 2021, 60 , 5527)。在此,我们解锁了裸 NbO x催化剂在氢分子解离和活化以及进一步氢解稳定的 C sp2 -C sp3中的能力模型化合物。原位漫反射红外傅里叶变换和密度泛函理论计算表明,由于表面氧空位的存在, H 2可以通过H 2的均裂和均裂裂解在Nb 2 O 5上解离并产生稳定的表面氢化物物种。此外,具有丰富氧空位和布朗斯台德酸度的 NbOPO 4催化剂不仅可以将苯基环己烷转化为单环化合物,而且可以通过裂解 C sp2 -C sp3键以高选择性将聚苯乙烯转化为芳烃。本研究提供并证明了 NbOPO 4的独特能力H 2的异裂和均裂以及坚固的C sp2 -C sp3键化合物(包括塑料)的氢解中的催化剂,这将有助于设计低成本的无金属氢解催化剂。
更新日期:2022-04-07
中文翻译:

Csp2-Csp3 键在无金属 NbOPO4 催化剂上的氢解裂解
Ru/NbO x催化剂结合了易于氢活化、与苯环的强结合能力以及存在布朗斯台德酸位点等优点,在利用木质素和芳香族化合物中对 C sp2 -C sp3键断裂进行了深入研究。塑料(Chem 2019, 5 , 1521;Angew. Chem. Int. Ed. 2021, 60 , 5527)。在此,我们解锁了裸 NbO x催化剂在氢分子解离和活化以及进一步氢解稳定的 C sp2 -C sp3中的能力模型化合物。原位漫反射红外傅里叶变换和密度泛函理论计算表明,由于表面氧空位的存在, H 2可以通过H 2的均裂和均裂裂解在Nb 2 O 5上解离并产生稳定的表面氢化物物种。此外,具有丰富氧空位和布朗斯台德酸度的 NbOPO 4催化剂不仅可以将苯基环己烷转化为单环化合物,而且可以通过裂解 C sp2 -C sp3键以高选择性将聚苯乙烯转化为芳烃。本研究提供并证明了 NbOPO 4的独特能力H 2的异裂和均裂以及坚固的C sp2 -C sp3键化合物(包括塑料)的氢解中的催化剂,这将有助于设计低成本的无金属氢解催化剂。

































 京公网安备 11010802027423号
京公网安备 11010802027423号