当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transformation of phytosterols into pregnatetraenedione by a combined microbial and chemical process
Green Chemistry ( IF 9.3 ) Pub Date : 2022-04-07 , DOI: 10.1039/d1gc04819h Yong-Jun Liu 1 , Wei-ting Ji 1 , Lu Song 1 , Xin-Yi Tao 1 , Ming Zhao 1 , Bei Gao 1 , Hao Meng 2 , Feng-Qing Wang 1 , Dong-Zhi Wei 1
Green Chemistry ( IF 9.3 ) Pub Date : 2022-04-07 , DOI: 10.1039/d1gc04819h Yong-Jun Liu 1 , Wei-ting Ji 1 , Lu Song 1 , Xin-Yi Tao 1 , Ming Zhao 1 , Bei Gao 1 , Hao Meng 2 , Feng-Qing Wang 1 , Dong-Zhi Wei 1
Affiliation
|
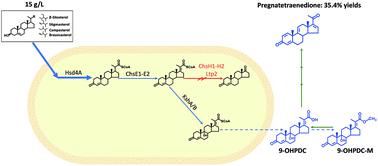
Pregna-1,4,9(11),16(17)-tetraene-3,20-dione (pregnatetraenedione) is the most useful precursor for the synthesis of corticosteroids in industry. However, the production of pregnatetraenedione is still a complex and expensive process. Here, we proposed a greener and more economical route for the synthesis of pregnatetraenedione, which was achieved via the conversion of phytosterols to an uncommon steroid compound, 9α-hydroxy-3-oxo-4,17(20)-pregna-diene-20-carboxylic acid (9-OHPDC), by an engineered Mycolicibacterium neoaurum. 9-OHPDC is a metabolite of sterols obtained by partially degrading its C17 side-chain and hitherto it is difficult to exclusively produce due to its complex metabolic process. In this study, we systematically investigated the determinants related to the generation of 9-OHPDC in the metabolic process of phytosterols and developed a 9-OHPDC-producing strain with the methyl-esterified 9-OHPDC (9-OHPDC-M) as the main product by metabolic engineering. The production of 9-OHPDC and 9-OHPDC-M respectively reached 9.86 g L−1 and 1.27 g L−1 in about 6 days and the total mole yield was more than 85.9%. Using 9-OHPDC-M as a synthon, subsequently, a four-step chemical process for the synthesis of pregnatetraenedione was designed and verified, which was composed of the following steps: hydrolysis of 9-OHPDC-M to 9-OHPDC, decarboxylation rearrangement, dehydration and Δ1 dehydrogenation. From phytosterols to pregnatetraenedione, the combined microbial and chemical process showed a total weight yield of up to 35.4%, significantly higher than that of the current industrial routes. In addition, compared with the current routes, the new synthesis route has the advantages of shorter reaction steps, milder reaction conditions, reduced use of organic solvents and abandonment of toxic catalysts. In a word, this study provided a green route for the production of pregnatetraenedione, which can be used for corticosteroid production in industry.
中文翻译:

通过微生物和化学相结合的方法将植物甾醇转化为孕四烯二酮
Pregna-1,4,9(11),16(17)-tetraene-3,20-dione (pregnatetraenedione) 是工业上合成皮质类固醇最有用的前体。然而,孕四烯二酮的生产仍然是一个复杂且昂贵的过程。在这里,我们提出了一种更环保、更经济的孕四烯二酮合成路线,该路线是通过将植物甾醇转化为一种罕见的类固醇化合物 9α-hydroxy-3-oxo-4,17(20)-pregna-diene-20 来实现的。 -羧酸 (9-OHPDC),由工程化的新金分枝杆菌. 9-OHPDC是甾醇的代谢产物,通过部分降解其C17侧链而获得,由于其复杂的代谢过程,迄今为止难以完全生产。在这项研究中,我们系统地研究了植物甾醇代谢过程中与 9-OHPDC 产生相关的决定因素,并开发了以甲酯化 9-OHPDC (9-OHPDC-M) 为主要产生 9-OHPDC 的菌株。代谢工程的产物。9-OHPDC和9-OHPDC-M的产量分别达到9.86 g L -1和1.27 g L -1约6天,总摩尔产率超过85.9%。以9-OHPDC-M为合成子,设计并验证了四步化学合成孕四烯二酮的工艺,该工艺由以下步骤组成:9-OHPDC-M水解为9-OHPDC,脱羧重排, 脱水和 Δ 1脱氢。从植物甾醇到孕四烯二酮,微生物和化学联合工艺的总重量产率高达35.4%,明显高于目前的工业路线。此外,与现有路线相比,新的合成路线具有反应步骤更短、反应条件更温和、有机溶剂用量减少、无需有毒催化剂等优点。总之,本研究为孕四烯二酮的生产提供了一条绿色途径,可用于工业生产皮质类固醇。
更新日期:2022-04-07
中文翻译:

通过微生物和化学相结合的方法将植物甾醇转化为孕四烯二酮
Pregna-1,4,9(11),16(17)-tetraene-3,20-dione (pregnatetraenedione) 是工业上合成皮质类固醇最有用的前体。然而,孕四烯二酮的生产仍然是一个复杂且昂贵的过程。在这里,我们提出了一种更环保、更经济的孕四烯二酮合成路线,该路线是通过将植物甾醇转化为一种罕见的类固醇化合物 9α-hydroxy-3-oxo-4,17(20)-pregna-diene-20 来实现的。 -羧酸 (9-OHPDC),由工程化的新金分枝杆菌. 9-OHPDC是甾醇的代谢产物,通过部分降解其C17侧链而获得,由于其复杂的代谢过程,迄今为止难以完全生产。在这项研究中,我们系统地研究了植物甾醇代谢过程中与 9-OHPDC 产生相关的决定因素,并开发了以甲酯化 9-OHPDC (9-OHPDC-M) 为主要产生 9-OHPDC 的菌株。代谢工程的产物。9-OHPDC和9-OHPDC-M的产量分别达到9.86 g L -1和1.27 g L -1约6天,总摩尔产率超过85.9%。以9-OHPDC-M为合成子,设计并验证了四步化学合成孕四烯二酮的工艺,该工艺由以下步骤组成:9-OHPDC-M水解为9-OHPDC,脱羧重排, 脱水和 Δ 1脱氢。从植物甾醇到孕四烯二酮,微生物和化学联合工艺的总重量产率高达35.4%,明显高于目前的工业路线。此外,与现有路线相比,新的合成路线具有反应步骤更短、反应条件更温和、有机溶剂用量减少、无需有毒催化剂等优点。总之,本研究为孕四烯二酮的生产提供了一条绿色途径,可用于工业生产皮质类固醇。































 京公网安备 11010802027423号
京公网安备 11010802027423号