Journal of Membrane Science ( IF 8.4 ) Pub Date : 2022-04-06 , DOI: 10.1016/j.memsci.2022.120448 Lincan Yang 1 , Zhiqian Wang 1 , Fanghui Wang 1 , Zhongming Wang 1 , Hong Zhu 1
|
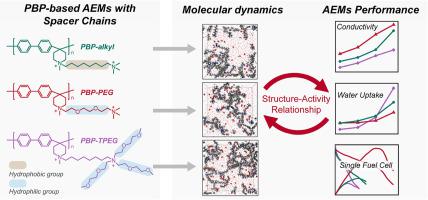
Anion-exchange membrane fuel cells (AEMFCs) is a promising solution to decrease the cost of fuel cell, because it is adaptable to non-noble-metals catalysts and low-cost stack components. However, anion exchange membranes (AEMs), a crucial component of AEMFCs devices, with desired properties (high ions conductivity, excellent chemical stability, robust mechanical strength, etc.) are currently unavailable. In this paper, poly(biphenyl N-methylpiperidine) (PBP) was synthesized as the backbones of AEMs, which could provide good chemical stability and robust mechanical strength. Quaternary ammonium cations containing various types of substituent sidechains, including hydrophobic alkyl chain, hydrophilic PEG chain and multi-PEG chains, were introduced onto the PBP backbones, and resulting AEMs were named PBP-alkyl, PBP-PEG and PBP-TPEG, respectively. The relationship between AEMs structure and performance were studied, and molecular dynamics (MD) simulations were carried out to microcosmically reveal the mechanism of structure-activity relationship. All the PBP-based AEMs have acceptable alkaline stability and performance loss is less than 10% under 2 M NaOH at 80 °C for about 500 h. PBP-PEG has the highest ions conductivity of 97.3 mS cm-1 and lowest water uptake (WU), while PBP-TPEG has the lowest ions conductivity of 56.1 mS cm-1 and highest WU. For single cell evaluations at 80 °C under H2/O2 condition, AEMFC containing PBP-PEG membrane bursts out a highest peak power density of 373 mW cm-2, while PBP-alkyl and PBP-TPEG only achieve 133 and 93 mW cm-2, respectively. MD results show the water channel of PBP-PEG is denser and more continuous than other structures, and the proportion of completely hydrated transport is higher than other structures, which contributes to the increase of overall performance. On the other hand, DFT results show the low basicity and strong hydrophily of cation containing multi-PEG substituents leads to the poor performance of PBP-TPEG. Therefore, for the design of AEMs molecular structures with outstanding performances, hydrophilic PEG spacer chain is more effective than hydrophobic alkyl spacer chain, and PEG is more effective as a spacer chain than as an extender chain.
中文翻译:

用于燃料电池的具有阳离子扩链剂侧链的聚芳基哌啶鎓阴离子交换膜
阴离子交换膜燃料电池(AEMFCs)是一种很有前途的降低燃料电池成本的解决方案,因为它适用于非贵金属催化剂和低成本的电堆组件。然而,目前还无法获得具有所需性能(高离子电导率、优异的化学稳定性、强大的机械强度等)的阴离子交换膜(AEM),它是 AEMFC 器件的关键组成部分。在本文中,合成了聚(联苯 N-甲基哌啶)(PBP)作为 AEM 的骨架,它可以提供良好的化学稳定性和强大的机械强度。将含有各种取代基侧链(包括疏水烷基链、亲水 PEG 链和多 PEG 链)的季铵阳离子引入 PBP 主链上,得到的 AEM 分别命名为 PBP-烷基、PBP-PEG 和 PBP-TPEG。研究了AEMs结构与性能之间的关系,并进行了分子动力学(MD)模拟,从微观上揭示了构效关系的机理。所有基于 PBP 的 AEM 都具有可接受的碱性稳定性,并且在 2 M NaOH 和 80 °C 约 500 小时下,性能损失小于 10%。PBP-PEG 具有 97.3 mS cm 的最高离子电导率-1和最低的吸水率 (WU),而 PBP-TPEG 的最低离子电导率为 56.1 mS cm -1和最高的 WU。对于 H 2 /O 2条件下 80 °C 的单细胞评估,含有 PBP-PEG 膜的 AEMFC 爆发出 373 mW cm -2的最高峰值功率密度,而 PBP-烷基和 PBP-TPEG 仅达到 133 和 93 mW厘米-2, 分别。MD结果表明,PBP-PEG的水通道比其他结构更密集、更连续,完全水合传输的比例高于其他结构,有助于提高整体性能。另一方面,DFT结果表明含有多PEG取代基的阳离子的低碱度和强亲水性导致PBP-TPEG性能较差。因此,对于性能优异的AEMs分子结构的设计,亲水性PEG间隔链比疏水性烷基间隔链更有效,PEG作为间隔链比作为延伸链更有效。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号