Journal of Power Sources ( IF 8.1 ) Pub Date : 2022-04-07 , DOI: 10.1016/j.jpowsour.2022.231395 Yujin Son 1 , Dung The Nguyen 2 , Youngil Lee 1, 2
|
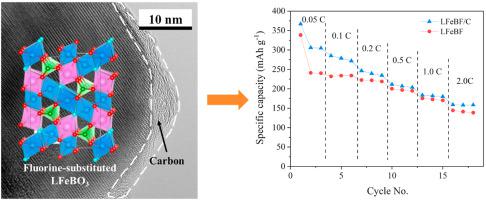
The fluorine-substituted LiFeBO3 with carbon coating (LFeBF/C) has been successfully demonstrated via a solid-state reaction to enhance its electrochemical properties and stability in air. Transmission electron microscopy characterization reveals that the primary LFeBF particles are fully covered by a thin layer of amorphous carbon. The formation of vonsenite-type LFeBF/C and the distribution of Li ions into its octahedral sites due to the fluorine substitution are confirmed using X-ray diffraction and solid-state nuclear magnetic resonance measurements, respectively. The stability of the LFeBF/C in air is promoted by the carbon layer. After two months of open-air storage, no significant changes in X-ray diffraction patterns can be observed. The synergistic effects of fluorine substitution and carbon coating enhance the overall electrochemical performance of the LFeBF/C as achieving a superior initial discharge capacity of 367.0 mAh g−1 at the rate of 0.05C and 231.7 mAh g−1 at 1.0C. In addition, the cyclic stability of the LFeBF/C is improved, reaching 197.5 mAh g−1 at the rate of 1.0C after 50 cycles, which corresponds to a retention of 85% of its initial capacity.
中文翻译:

具有碳涂层的氟取代 LiFeBO3 的制备和表征,以提高作为锂离子电池正极材料的电化学性能和稳定性
氟取代的 LiFeBO 3碳涂层 (LFeBF/C) 已通过固态反应成功地证明,以增强其在空气中的电化学性能和稳定性。透射电子显微镜表征表明,初级 LFeBF 颗粒完全被一层薄薄的无定形碳覆盖。分别使用 X 射线衍射和固态核磁共振测量证实了方铅矿型 LFeBF/C 的形成以及由于氟取代而导致的锂离子分布到其八面体位点中。碳层促进了 LFeBF/C 在空气中的稳定性。在露天储存两个月后,X 射线衍射图没有明显变化。-1 在 0.05C 的速率和 231.7 mAh g -1 在 1.0C 的速率。此外,LFeBF/C 的循环稳定性得到改善,50 次循环后以 1.0C 的速率达到 197.5 mAh g -1 ,相当于保留了 85% 的初始容量。































 京公网安备 11010802027423号
京公网安备 11010802027423号