Journal of Energy Chemistry ( IF 14.0 ) Pub Date : 2022-04-06 , DOI: 10.1016/j.jechem.2022.03.046 Kanghui Hu 1, 2 , Li Ren 1 , Weifeng Fan 3 , Bing Zhang 3 , Meihua Zuo 3 , Yanhui Zhang 3 , Genpin Lv 4 , Huiyuan Xu 5 , Wei Xiang 1, 3 , Xiaodong Guo 2
|
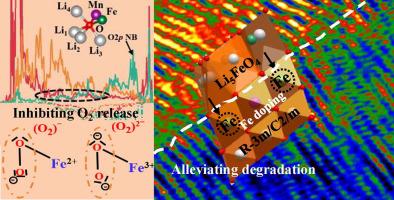
Li-rich layered transition metal oxides are one of the most promising cathode materials for their high energy density. However, the cathodes usually suffer from severe potential dropping and capacity fading during cycling, which are associated with the surface oxygen release and accompanied by cation densification and structural collapse. Herein, an integrative approach of simultaneous constructing uniform 3d Fe-ion doping in the transition metal layer and Li-rich Li5FeO4 shell to grab the oxygen and prevent interfacial side reactions is proposed. The introduction of Fe induces higher redox potential and stronger 3d Fe-O 2p covalent bond, triggering reversible anionic redox via a reductive coupling mechanism. And the delithiated product of Li-rich Li5FeO4 not only acts as a protective layer alleviating the side reactions but also enhances the surface kinetic property. With the benefit of promoted reversibility of oxygen redox and enhanced surface stability, the cathode exhibits high reversible capacity and superior cycle performance. Density function theory calculation indicates that the O 2p non-bonding state in the cathode incorporated with Fe sits at a lower energy band, resulting in higher energy storage voltage and improved oxygen stability. Consequently, the modified cathode exhibits a discharge specific capacity of 307 mA h g−1 (1C = 250 mA g−1), coulombic efficiency of 82.09% in the initial cycle at 0.1C and 88.34% capacity retention after 100 cycles at 1C. The work illustrates a strategy that could simultaneously enhance oxygen redox reversibility and interface stability by constructing lattice bond coordination and delithiation induced protective layer to develop Li-rich materials with high reversible capacity and long lifespan.
中文翻译:

通过脱锂诱导保护层和 Fe-O 配位调节氧化还原活性,提高富锂正极的电压和循环性能
富锂层状过渡金属氧化物因其高能量密度而成为最有前途的正极材料之一。然而,正极在循环过程中通常会遭受严重的电位下降和容量衰减,这与表面氧释放有关,并伴有阳离子致密化和结构坍塌。在此,提出了一种在过渡金属层和富锂Li 5 FeO 4壳层中同时构建均匀的3 d Fe离子掺杂以抓住氧气并防止界面副反应的综合方法。Fe的引入导致更高的氧化还原电位和更强的3 d Fe-O 2 p共价键,通过还原偶联机制触发可逆的阴离子氧化还原。富锂Li 5 FeO 4的脱锂产物不仅起到保护层的作用,减轻了副反应,而且提高了表面动力学性能。由于促进了氧氧化还原的可逆性和增强的表面稳定性,正极表现出高可逆容量和优异的循环性能。密度泛函理论计算表明,掺入Fe的正极中的O 2 p非键态处于较低的能带,从而提高了储能电压,提高了氧的稳定性。因此,改性正极的放电比容量为 307 mA h g -1 (1C = 250 mA g-1 ),在 0.1C 的初始循环中库仑效率为 82.09%,在 1C 循环 100 次后容量保持率为 88.34%。该工作说明了一种策略,可以通过构建晶格键配位和脱锂诱导保护层来同时提高氧氧化还原可逆性和界面稳定性,从而开发出具有高可逆容量和长寿命的富锂材料。






























 京公网安备 11010802027423号
京公网安备 11010802027423号