当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Benzophenone vs. Copper/Benzophenone in Light‐Promoted Atom Transfer Radical Additions (ATRAs): Highly Effective Iodoperfluoroalkylation of Alkenes/Alkynes and Mechanistic Studies
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2016-08-31 , DOI: 10.1002/adsc.201600501 Redouane Beniazza 1 , Rachel Atkinson 1 , Christelle Absalon 1 , Frédéric Castet 1 , Sergey A. Denisov 1 , Nathan D. McClenaghan 1 , Dominique Lastécouères 1 , Jean-Marc Vincent 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2016-08-31 , DOI: 10.1002/adsc.201600501 Redouane Beniazza 1 , Rachel Atkinson 1 , Christelle Absalon 1 , Frédéric Castet 1 , Sergey A. Denisov 1 , Nathan D. McClenaghan 1 , Dominique Lastécouères 1 , Jean-Marc Vincent 1
Affiliation
|
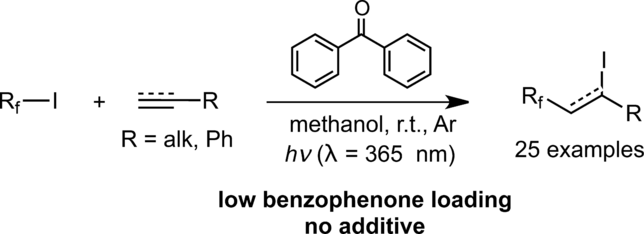
Iodoperfluooralkylation of terminal alkenes and alkynes is effectively photo‐promoted by benzophenone 2 (BP) or the photoreducible copper(II) complex 1. In particular, BP at 1 mol% in methanol upon 365 nm irradiation with a low‐pressure mercury lamp (type TLC=thin layer chromatography, 6 W) results in a fast reaction with excellent reaction yields. Complex 1 and BP 2 exhibited very similar reactivity, suggesting that the reactions involving 1 are likely to be governed by the benzophenone photoactivation processes, rather than copper(I)/(II) redox processes. Mechanistic investigations using transient absorption spectroscopy revealed that a deactivation pathway of the benzophenone triplet (3BP*) is via its reaction with the methanol solvent. We propose that the generated radicals, in particular .CH2OH, play a key role in the initiation step forming Rf. by reacting with RfI, Rf. then entering a radical chain cycle. 1H NMR studies provided evidence that a substantial amount (∼7% NMR yield) of the hemiacetal CH3OCH2OH is formed, i.e., the possible by‐product of the reaction between .CH2OH and RfI. Finally, DFT calculations indicate that a triplet‐triplet energy transfer (TTET) process from 3BP* to perfluorooctyl iodide (C8F17I) is unlikely or should be rather slow under the reaction conditions, consistent with the transient absorption studies.
中文翻译:

光促进的原子转移自由基加成(ATRA)中的苯甲酮与铜/苯甲酮:烯烃/炔烃的高效碘全氟烷基化及机理研究
二苯甲酮2(BP)或光还原性铜(II)络合物1有效促进了末端烯烃和炔烃的碘过氟烷基化。尤其是,在低压汞灯(TLC =薄层色谱,6 W)照射365 nm时,甲醇中1摩尔%的BP可以实现快速反应,并具有出色的反应产率。配合物1和BP 2表现出非常相似的反应性,表明涉及1的反应很可能是由二苯甲酮的光活化过程而不是铜(I)/(II)氧化还原过程控制的。使用瞬态吸收光谱的机理研究表明,二苯甲酮三联体的失活途径(3BP *)是通过其与甲醇溶剂的反应来进行的。我们建议特别是生成的部首。CH 2 OH在形成R f的引发步骤中起关键作用。通过与R f I,R f反应。然后进入一个彻底的连锁循环。1 H NMR研究提供了证据,表明形成了大量(约7%NMR)半缩醛CH 3 OCH 2 OH,即之间可能发生的副产物。CH 2 OH和R ˚F I.最后,DFT计算表明,从三线态-三线态能量传递(TTET)工艺与瞬态吸收研究一致,在反应条件下,将3 BP *转化为全氟辛基碘化物(C 8 F 17 I)的可能性不大,或者应该相当缓慢。
更新日期:2016-08-31
中文翻译:

光促进的原子转移自由基加成(ATRA)中的苯甲酮与铜/苯甲酮:烯烃/炔烃的高效碘全氟烷基化及机理研究
二苯甲酮2(BP)或光还原性铜(II)络合物1有效促进了末端烯烃和炔烃的碘过氟烷基化。尤其是,在低压汞灯(TLC =薄层色谱,6 W)照射365 nm时,甲醇中1摩尔%的BP可以实现快速反应,并具有出色的反应产率。配合物1和BP 2表现出非常相似的反应性,表明涉及1的反应很可能是由二苯甲酮的光活化过程而不是铜(I)/(II)氧化还原过程控制的。使用瞬态吸收光谱的机理研究表明,二苯甲酮三联体的失活途径(3BP *)是通过其与甲醇溶剂的反应来进行的。我们建议特别是生成的部首。CH 2 OH在形成R f的引发步骤中起关键作用。通过与R f I,R f反应。然后进入一个彻底的连锁循环。1 H NMR研究提供了证据,表明形成了大量(约7%NMR)半缩醛CH 3 OCH 2 OH,即之间可能发生的副产物。CH 2 OH和R ˚F I.最后,DFT计算表明,从三线态-三线态能量传递(TTET)工艺与瞬态吸收研究一致,在反应条件下,将3 BP *转化为全氟辛基碘化物(C 8 F 17 I)的可能性不大,或者应该相当缓慢。

































 京公网安备 11010802027423号
京公网安备 11010802027423号