当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Patent Review on FDA-Approved Antibody-Drug Conjugates, Their Linkers and Drug Payloads
ChemMedChem ( IF 3.6 ) Pub Date : 2022-04-05 , DOI: 10.1002/cmdc.202200032 C S Brian Chia 1
ChemMedChem ( IF 3.6 ) Pub Date : 2022-04-05 , DOI: 10.1002/cmdc.202200032 C S Brian Chia 1
Affiliation
|
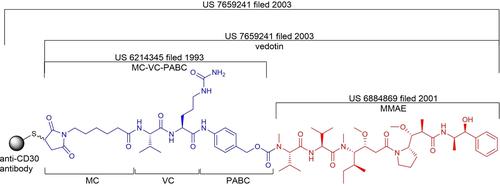
Antibody-drug conjugates (ADCs) have proven to be a promising class of biologics to treat various cancers. Unlike small-molecule drugs, ADCs are structurally much more complex and hence, protected by more complex patent landscapes. This review collates the patents protecting eleven approved ADCs up to 31 December 2021, with particular emphasis on their linkers and toxin payloads.
中文翻译:

FDA 批准的抗体-药物偶联物、其接头和药物有效载荷的专利审查
抗体-药物偶联物(ADC) 已被证明是治疗各种癌症的一类有前途的生物制剂。与小分子药物不同,ADC 在结构上要复杂得多,因此受到更复杂的专利环境的保护。该审查整理了截至 2021 年 12 月 31 日保护 11 个获批 ADC 的专利,特别强调了它们的接头和毒素有效载荷。
更新日期:2022-04-05
中文翻译:

FDA 批准的抗体-药物偶联物、其接头和药物有效载荷的专利审查
抗体-药物偶联物(ADC) 已被证明是治疗各种癌症的一类有前途的生物制剂。与小分子药物不同,ADC 在结构上要复杂得多,因此受到更复杂的专利环境的保护。该审查整理了截至 2021 年 12 月 31 日保护 11 个获批 ADC 的专利,特别强调了它们的接头和毒素有效载荷。































 京公网安备 11010802027423号
京公网安备 11010802027423号