当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-Catalyzed Synthesis of an Aryl Nitrile via Aryl Exchange between an Aromatic Amide and a Simple Nitrile
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-04-05 , DOI: 10.1021/acscatal.2c01029
Yang Long 1 , Yanling Zheng 1 , Ying Xia 2 , Lang Qu 1 , Yuhe Yang 1 , Haifeng Xiang 1 , Xiangge Zhou 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-04-05 , DOI: 10.1021/acscatal.2c01029
Yang Long 1 , Yanling Zheng 1 , Ying Xia 2 , Lang Qu 1 , Yuhe Yang 1 , Haifeng Xiang 1 , Xiangge Zhou 1
Affiliation
|
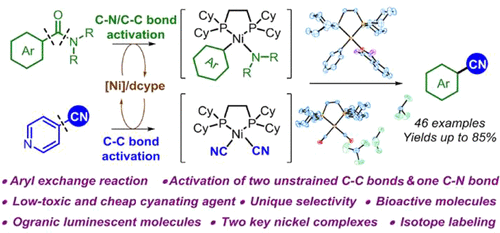
Herein, a nickel-catalyzed synthesis of an aryl nitrile via aryl exchange between an aromatic amide and a simple nitrile was developed. By using cheap, easy-to-handle, and low-toxic 4-cyanopyridine as the cyanating source, cyanation of various aromatic amides afforded an assortment of aryl nitriles including bioactive drugs and organic luminescent molecules in good yields. The reaction exhibited wide substrate scope, good functional group tolerance, and unique selectivity that were complementary to traditional methods. Moreover, two key nickel complexes formed by oxidative addition to each substrate are obtained and determined by X-ray crystallography, which gave strong support for the mechanism elucidations.
中文翻译:

通过芳族酰胺和简单腈之间的芳基交换,镍催化合成芳基腈
在此,开发了一种通过芳族酰胺和简单腈之间的芳基交换,在镍催化下合成芳基腈的方法。通过使用廉价、易于处理和低毒的 4-氰基吡啶作为氰化源,各种芳香酰胺的氰化反应以良好的收率提供了包括生物活性药物和有机发光分子在内的各种芳基腈。该反应具有广泛的底物范围、良好的官能团耐受性和独特的选择性,与传统方法互补。此外,通过X射线晶体学获得并确定了通过氧化添加到每个基材中形成的两个关键镍配合物,这为机理阐明提供了有力支持。
更新日期:2022-04-05
中文翻译:

通过芳族酰胺和简单腈之间的芳基交换,镍催化合成芳基腈
在此,开发了一种通过芳族酰胺和简单腈之间的芳基交换,在镍催化下合成芳基腈的方法。通过使用廉价、易于处理和低毒的 4-氰基吡啶作为氰化源,各种芳香酰胺的氰化反应以良好的收率提供了包括生物活性药物和有机发光分子在内的各种芳基腈。该反应具有广泛的底物范围、良好的官能团耐受性和独特的选择性,与传统方法互补。此外,通过X射线晶体学获得并确定了通过氧化添加到每个基材中形成的两个关键镍配合物,这为机理阐明提供了有力支持。

































 京公网安备 11010802027423号
京公网安备 11010802027423号