当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics and Reaction Mechanisms of Acetic Acid Hydrodeoxygenation over Pt and Pt–Mo Catalysts
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-04-05 , DOI: 10.1021/acssuschemeng.2c00179 Yiteng Zheng 1 , Yue Qi 1 , Ziyu Tang 1 , Felix Hanke 2 , Simon G. Podkolzin 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-04-05 , DOI: 10.1021/acssuschemeng.2c00179 Yiteng Zheng 1 , Yue Qi 1 , Ziyu Tang 1 , Felix Hanke 2 , Simon G. Podkolzin 1
Affiliation
|
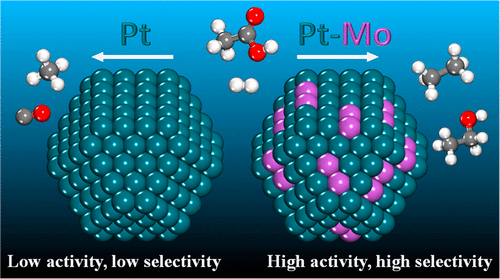
Kinetic measurements for silica-supported Pt and Pt–Mo catalysts were collected in vapor-phase acetic acid hydrodeoxygenation by varying the hydrogen partial pressure between 18 and 72 kPa and the acetic acid partial pressure between 7 and 18 kPa at 423–473 K. Under all testing conditions, the Pt–Mo catalyst was more active and selective. In addition, the apparent activation energy for Pt–Mo of 76 ± 3 kJ/mol was lower than that of 84 ± 4 kJ/mol for Pt. The apparent reaction orders were also different. The order in hydrogen of 0.8 ± 0.1 for Pt–Mo changed to zero at higher hydrogen partial pressures while that for Pt remained constant at 0.6 ± 0.1. A near-zero order in acetic acid for Pt–Mo changed to −2.1 at higher acetic acid pressures while that for Pt remained constant at −2.9 ± 0.3. These differences in reaction kinetics as well as in selectivity trends with changes in temperature and feed composition indicated a change in the reaction mechanism for Pt–Mo. The catalysts were characterized with hydrogen temperature programmed desorption, oxygen temperature programmed oxidation and transmission electron microscopy with energy-dispersed X-ray spectroscopy elemental mapping. Mo was present in the form of subnanometer-size clusters on the surface of Pt nanoparticles. Both Pt and Pt–Mo catalysts were stable under the reaction conditions for 10 h, and the size and structure of Pt and Pt–Mo particles remained mostly unchanged, without coke accumulation. Density functional theory calculations show that neighboring Pt–Mo surface atoms act as a single active site where Mo serves as a preferential binding anchor for O atoms. The presence of Mo changes the structure and reactivity of hydrocarbon surface species, leading to a change in the reaction mechanism. In contrast with Pt, C–O bond breaking reactions become more favorable on Pt–Mo and, conversely, C–C bond breaking reactions become less favorable on Pt–Mo, explaining the experimentally observed higher activities and selectivities of Pt–Mo.
中文翻译:

Pt和Pt-Mo催化剂上乙酸加氢脱氧的动力学和反应机理
二氧化硅负载的 Pt 和 Pt-Mo 催化剂的动力学测量是在气相乙酸加氢脱氧中收集的,方法是在 423-473 K 下改变 18 和 72 kPa 之间的氢分压和 7 和 18 kPa 之间的乙酸分压。在所有测试条件下,Pt-Mo 催化剂的活性和选择性都更高。此外,Pt-Mo 的 76 ± 3 kJ/mol 的表观活化能低于 Pt 的 84 ± 4 kJ/mol。明显的反应顺序也不同。Pt-Mo 的 0.8 ± 0.1 的氢顺序在较高的氢分压下变为零,而 Pt 的氢分压保持在 0.6 ± 0.1 不变。在较高的乙酸压力下,Pt-Mo 的乙酸接近零级变为 -2.1,而 Pt 的乙酸保持恒定在 -2.9 ± 0.3。这些反应动力学以及选择性趋势随温度和进料组成变化的差异表明 Pt-Mo 的反应机理发生了变化。催化剂通过氢气程序升温脱附、氧气程序升温氧化和透射电子显微镜以及能量色散X射线光谱元素映射进行了表征。Mo以亚纳米级簇的形式存在于Pt纳米颗粒的表面。Pt 和 Pt-Mo 催化剂在反应条件下均稳定 10 h,Pt 和 Pt-Mo 颗粒的尺寸和结构基本保持不变,没有积炭。密度泛函理论计算表明,相邻的 Pt-Mo 表面原子充当单个活性位点,其中 Mo 充当 O 原子的优先结合锚。Mo的存在改变了烃表面物质的结构和反应性,导致反应机理发生变化。与 Pt 相比,C-O 键断裂反应对 Pt-Mo 更有利,相反,C-C 键断裂反应对 Pt-Mo 不利,这解释了实验观察到的 Pt-Mo 更高的活性和选择性。
更新日期:2022-04-05
中文翻译:

Pt和Pt-Mo催化剂上乙酸加氢脱氧的动力学和反应机理
二氧化硅负载的 Pt 和 Pt-Mo 催化剂的动力学测量是在气相乙酸加氢脱氧中收集的,方法是在 423-473 K 下改变 18 和 72 kPa 之间的氢分压和 7 和 18 kPa 之间的乙酸分压。在所有测试条件下,Pt-Mo 催化剂的活性和选择性都更高。此外,Pt-Mo 的 76 ± 3 kJ/mol 的表观活化能低于 Pt 的 84 ± 4 kJ/mol。明显的反应顺序也不同。Pt-Mo 的 0.8 ± 0.1 的氢顺序在较高的氢分压下变为零,而 Pt 的氢分压保持在 0.6 ± 0.1 不变。在较高的乙酸压力下,Pt-Mo 的乙酸接近零级变为 -2.1,而 Pt 的乙酸保持恒定在 -2.9 ± 0.3。这些反应动力学以及选择性趋势随温度和进料组成变化的差异表明 Pt-Mo 的反应机理发生了变化。催化剂通过氢气程序升温脱附、氧气程序升温氧化和透射电子显微镜以及能量色散X射线光谱元素映射进行了表征。Mo以亚纳米级簇的形式存在于Pt纳米颗粒的表面。Pt 和 Pt-Mo 催化剂在反应条件下均稳定 10 h,Pt 和 Pt-Mo 颗粒的尺寸和结构基本保持不变,没有积炭。密度泛函理论计算表明,相邻的 Pt-Mo 表面原子充当单个活性位点,其中 Mo 充当 O 原子的优先结合锚。Mo的存在改变了烃表面物质的结构和反应性,导致反应机理发生变化。与 Pt 相比,C-O 键断裂反应对 Pt-Mo 更有利,相反,C-C 键断裂反应对 Pt-Mo 不利,这解释了实验观察到的 Pt-Mo 更高的活性和选择性。































 京公网安备 11010802027423号
京公网安备 11010802027423号