当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bis(imino)acenaphthene (BIAN)-Supported N-Heterocyclic Carbene Palladium Complexes with Ancillary Ligands: Readily Activated Precatalysts for Direct C–H Arylation of Thiophenes
Organometallics ( IF 2.5 ) Pub Date : 2022-04-05 , DOI: 10.1021/acs.organomet.2c00007 Di-Zhong Zheng 1 , Dong-Hui Li 1 , Huan Liu 1 , Youxiang Shao 2 , Zhuofeng Ke 2 , Feng-Shou Liu 1
Organometallics ( IF 2.5 ) Pub Date : 2022-04-05 , DOI: 10.1021/acs.organomet.2c00007 Di-Zhong Zheng 1 , Dong-Hui Li 1 , Huan Liu 1 , Youxiang Shao 2 , Zhuofeng Ke 2 , Feng-Shou Liu 1
Affiliation
|
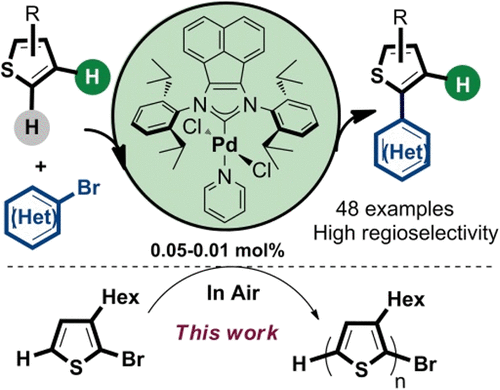
We report herein a highly efficient direct C–H arylation of thiophenes with (hetero)aryl bromides by bulky bis(imino)acenaphthene (BIAN)-supported N-heterocyclic carbene palladium complexes. The relationship between the structure of palladium complexes with ancillary ligands and catalytic properties was discussed. Upon a low palladium loading of 0.01–0.05 mol %, the bulky palladium complex was successfully used to catalyze the cross-coupling of a variety of thiophens with (hetero)aryl bromides under aerobic conditions. Furthermore, it provides a practical and straightforward access to poly(3-hexylthiophenes) with high molecular weight and high HT value under aerobic reaction conditions. To access the mechanistic of the transformation, experiment investigation and DFT calculations on the direct arylation were performed, which supported the involvement of a Pd(0)/Pd(II) CMD process.
中文翻译:

双(亚氨基)苊 (BIAN) 负载的具有辅助配体的 N-杂环卡宾钯配合物:用于噻吩直接 C-H 芳基化的易活化预催化剂
我们在此报告了通过庞大的双(亚氨基)苊(BIAN)负载的N将噻吩与(杂)芳基溴化物的高效直接 C-H 芳基化-杂环卡宾钯配合物。讨论了钯与辅助配体的结构与催化性能之间的关系。在 0.01-0.05 mol% 的低钯负载量下,庞大的钯络合物成功地用于在有氧条件下催化各种噻吩与(杂)芳基溴化物的交叉偶联。此外,它提供了一种在好氧反应条件下获得具有高分子量和高 HT 值的聚 (3-己基噻吩) 的实用且直接的途径。为了了解转化机制,对直接芳基化进行了实验研究和 DFT 计算,这支持了 Pd (0) /Pd (II) CMD 过程的参与。
更新日期:2022-04-05
中文翻译:

双(亚氨基)苊 (BIAN) 负载的具有辅助配体的 N-杂环卡宾钯配合物:用于噻吩直接 C-H 芳基化的易活化预催化剂
我们在此报告了通过庞大的双(亚氨基)苊(BIAN)负载的N将噻吩与(杂)芳基溴化物的高效直接 C-H 芳基化-杂环卡宾钯配合物。讨论了钯与辅助配体的结构与催化性能之间的关系。在 0.01-0.05 mol% 的低钯负载量下,庞大的钯络合物成功地用于在有氧条件下催化各种噻吩与(杂)芳基溴化物的交叉偶联。此外,它提供了一种在好氧反应条件下获得具有高分子量和高 HT 值的聚 (3-己基噻吩) 的实用且直接的途径。为了了解转化机制,对直接芳基化进行了实验研究和 DFT 计算,这支持了 Pd (0) /Pd (II) CMD 过程的参与。






























 京公网安备 11010802027423号
京公网安备 11010802027423号