当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sulfur-Doped g-C3N4-Supported Ni Species with a Wide Temperature Window for Acetylene Semihydrogenation
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-04-04 , DOI: 10.1021/acssuschemeng.1c07263
Huiran Zhou 1 , Bingxin Li 1 , Huigen Fu 1 , Xiaohua Zhao 1 , Mingming Zhang 1 , Xiaobing Wang 1 , Yang Liu 1 , Zongxian Yang 2 , Xiangdong Lou 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-04-04 , DOI: 10.1021/acssuschemeng.1c07263
Huiran Zhou 1 , Bingxin Li 1 , Huigen Fu 1 , Xiaohua Zhao 1 , Mingming Zhang 1 , Xiaobing Wang 1 , Yang Liu 1 , Zongxian Yang 2 , Xiangdong Lou 1
Affiliation
|
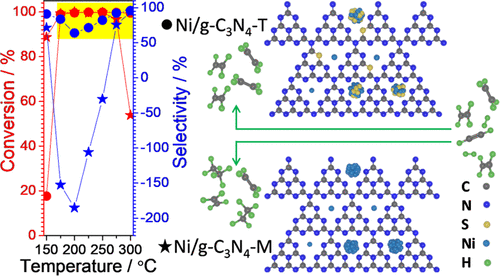
The semihydrogenation of acetylene is a vital industrial reaction in C2 hydrorefining. Ethylene selectivity and hydrogenation activity, however, are difficult to optimize simultaneously, naturally leading to a narrow operation temperature window, especially for noble-metal-free catalysts, which are significant for sustainable development. Herein, Ni/g-C3N4-T, i.e., sulfur-doped g-C3N4-T-supported Ni species, is proved to be an excellent Ni catalyst for acetylene semihydrogenation. Full conversion and good selectivity (>63%) are achieved concurrently over Ni/g-C3N4-T in a very wide operation window of 175–300 °C, in sharp contrast to the negative selectivity of Ni/g-C3N4-M, i.e., Ni supported on sulfur-free g-C3N4-M. That is, the introduction of sulfur exerts a significant influence on the catalytic behavior of Ni/g-C3N4 for acetylene hydrogenation. The results of X-ray diffraction (XRD), high-resolution transmission electron microscopy (HRTEM), energy-dispersive X-ray spectroscopy (EDS), and X-ray photoelectron spectroscopy (XPS) indicate that S-doped g-C3N4-T-supported Ni species exist as Ni cations confined in the cavities of g-C3N4-T or in the form of a Ni-S solid solution with S surface segregation, different from Ni particles for Ni/g-C3N4-M. Accordingly, the continuous Ni ensembles, at least the surface counterparts, are broken up, which, as indicated by C2H4-temperature-programmed desorption (TPD), will promote desorption of weak π-bonded ethylene; therefore, Ni/g-C3N4-T possesses high selectivity. In addition, exclusive Ni2+ species over g-C3N4-T, demonstrated by XPS, will favor the activation of acetylene via their electrostatic interaction; thus, Ni/g-C3N4-T shows a comparable hydrogenation activity with that of Ni/g-C3N4-M. The findings offer an avenue to design cost-effective catalysts with both high selectivity and superior activity over a broad operation window for acetylene semihydrogenation.
中文翻译:

用于乙炔半氢化的具有宽温度窗口的硫掺杂 g-C3N4 负载 Ni 物种
乙炔的半加氢反应是C 2加氢精制中一个重要的工业反应。然而,乙烯选择性和加氢活性难以同时优化,自然导致操作温度窗口狭窄,特别是对于无贵金属催化剂而言,这对于可持续发展具有重要意义。在此,Ni/gC 3 N 4 -T,即硫掺杂的gC 3 N 4 -T负载的Ni物质,被证明是乙炔半加氢的优良Ni催化剂。在 Ni/gC 3 N 4上同时实现完全转化和良好的选择性 (>63%)-T 在 175-300 °C 的非常宽的操作窗口中,与 Ni/gC 3 N 4 -M的负选择性形成鲜明对比,即 Ni 负载在无硫 gC 3 N 4 -M 上。也就是说,硫的引入对Ni/gC 3 N 4对乙炔加氢的催化行为有显着影响。X射线衍射(XRD)、高分辨率透射电子显微镜(HRTEM)、能量色散X射线光谱(EDS)和X射线光电子能谱(XPS)的结果表明,S掺杂的gC 3 N 4 -T-负载的Ni物种作为Ni阳离子存在于gC 3 N 4的空腔中-T或具有S表面偏析的Ni-S固溶体形式,不同于Ni/gC 3 N 4 -M的Ni颗粒。因此,连续的 Ni 集合体,至少是表面对应物被分解,如 C 2 H 4 -程序升温脱附 (TPD) 所示,这将促进弱 π 键乙烯的脱附;因此,Ni/gC 3 N 4 -T具有高选择性。此外, XPS 证明, gC 3 N 4 -T 上的独有 Ni 2+物质将有利于通过它们的静电相互作用激活乙炔;因此,Ni/gC 3 N 4-T显示出与Ni/gC 3 N 4 -M相当的加氢活性。这些发现为设计具有高选择性和优异活性的具有成本效益的催化剂提供了一条途径,该催化剂在乙炔半加氢的广泛操作窗口中。
更新日期:2022-04-04
中文翻译:

用于乙炔半氢化的具有宽温度窗口的硫掺杂 g-C3N4 负载 Ni 物种
乙炔的半加氢反应是C 2加氢精制中一个重要的工业反应。然而,乙烯选择性和加氢活性难以同时优化,自然导致操作温度窗口狭窄,特别是对于无贵金属催化剂而言,这对于可持续发展具有重要意义。在此,Ni/gC 3 N 4 -T,即硫掺杂的gC 3 N 4 -T负载的Ni物质,被证明是乙炔半加氢的优良Ni催化剂。在 Ni/gC 3 N 4上同时实现完全转化和良好的选择性 (>63%)-T 在 175-300 °C 的非常宽的操作窗口中,与 Ni/gC 3 N 4 -M的负选择性形成鲜明对比,即 Ni 负载在无硫 gC 3 N 4 -M 上。也就是说,硫的引入对Ni/gC 3 N 4对乙炔加氢的催化行为有显着影响。X射线衍射(XRD)、高分辨率透射电子显微镜(HRTEM)、能量色散X射线光谱(EDS)和X射线光电子能谱(XPS)的结果表明,S掺杂的gC 3 N 4 -T-负载的Ni物种作为Ni阳离子存在于gC 3 N 4的空腔中-T或具有S表面偏析的Ni-S固溶体形式,不同于Ni/gC 3 N 4 -M的Ni颗粒。因此,连续的 Ni 集合体,至少是表面对应物被分解,如 C 2 H 4 -程序升温脱附 (TPD) 所示,这将促进弱 π 键乙烯的脱附;因此,Ni/gC 3 N 4 -T具有高选择性。此外, XPS 证明, gC 3 N 4 -T 上的独有 Ni 2+物质将有利于通过它们的静电相互作用激活乙炔;因此,Ni/gC 3 N 4-T显示出与Ni/gC 3 N 4 -M相当的加氢活性。这些发现为设计具有高选择性和优异活性的具有成本效益的催化剂提供了一条途径,该催化剂在乙炔半加氢的广泛操作窗口中。




































 京公网安备 11010802027423号
京公网安备 11010802027423号