当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient one-step synthesis of 3-(indol-2-yl)quinoxalin-2(1H)-ones via electrochemical oxidative cross-dehydrogenative coupling
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2022-03-30 , DOI: 10.1039/d2nj00205a Hao Zhang 1 , Lishan Hu 1 , Kai Yu 2 , Lan-Lan Lou 1 , Shuangxi Liu 1
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2022-03-30 , DOI: 10.1039/d2nj00205a Hao Zhang 1 , Lishan Hu 1 , Kai Yu 2 , Lan-Lan Lou 1 , Shuangxi Liu 1
Affiliation
|
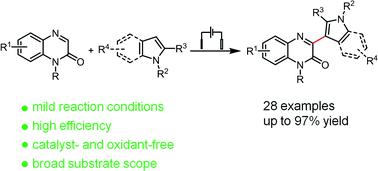
Developing an efficient and convenient method for direct synthesis of bioactive 3-(indol-2-yl)quinoxalin-2(1H)-ones is highly desirable in the pharmaceutical industry. In this work, 3-(indol-2-yl)quinoxalin-2(1H)-ones were synthesized by a one-step electrochemical cross-dehydrogenative coupling process from quinoxalin-2(1H)-ones and indoles. This protocol is simple, operationally convenient, and compatible with a broad range of substrates, enabling the synthesis of the desired coupling products in good to excellent yields (up to 97%) without the use of any catalyst or chemical oxidant. In addition, a reaction mechanism based on the electrochemical oxidation of indoles was proposed.
中文翻译:

通过电化学氧化交叉脱氢偶联高效一步合成 3-(indol-2-yl)quinoxalin-2(1H)-ones
开发一种直接合成具有生物活性的 3-(indol-2-yl)quinoxalin-2(1 H )-ones的高效便捷的方法在制药行业是非常需要的。在这项工作中,3-(indol-2-yl)quinoxalin-2(1 H )-ones 由 quinoxalin-2( 1H )-ones 和吲哚通过一步电化学交叉脱氢偶联过程合成。该协议简单、操作方便且与广泛的底物兼容,能够在不使用任何催化剂或化学氧化剂的情况下以良好至优异的产率(高达 97%)合成所需的偶联产物。此外,还提出了基于吲哚电化学氧化的反应机理。
更新日期:2022-03-30
中文翻译:

通过电化学氧化交叉脱氢偶联高效一步合成 3-(indol-2-yl)quinoxalin-2(1H)-ones
开发一种直接合成具有生物活性的 3-(indol-2-yl)quinoxalin-2(1 H )-ones的高效便捷的方法在制药行业是非常需要的。在这项工作中,3-(indol-2-yl)quinoxalin-2(1 H )-ones 由 quinoxalin-2( 1H )-ones 和吲哚通过一步电化学交叉脱氢偶联过程合成。该协议简单、操作方便且与广泛的底物兼容,能够在不使用任何催化剂或化学氧化剂的情况下以良好至优异的产率(高达 97%)合成所需的偶联产物。此外,还提出了基于吲哚电化学氧化的反应机理。






























 京公网安备 11010802027423号
京公网安备 11010802027423号