当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Incorporating Oxygen Atoms in a SnS2 Atomic Layer to Simultaneously Stabilize Atomic Hydrogen and Accelerate the Generation of Hydroxyl Radicals for Water Decontamination
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2022-03-29 , DOI: 10.1021/acs.est.1c07152 Shanpeng Li 1 , Chunlei Liu 2 , Wenying Lv 1 , Guoguang Liu 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2022-03-29 , DOI: 10.1021/acs.est.1c07152 Shanpeng Li 1 , Chunlei Liu 2 , Wenying Lv 1 , Guoguang Liu 1
Affiliation
|
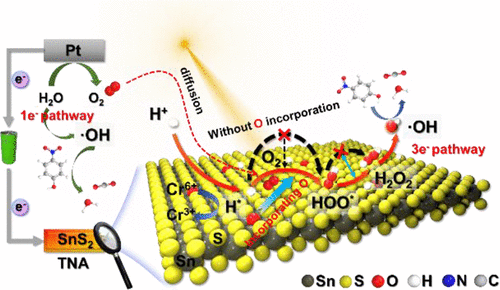
Photoelectrocatalysis (PEC) is an efficient way to address various pollutants. Surface-adsorbed atomic hydrogen (H*) and hydroxyl radicals (•OH) play a key role in the PEC process. However, the instability of H* and low production of •OH considerably limit the PEC efficiency. In this study, we noted that incorporating oxygen atoms could regulate the behavior of H* by creating a locally favorable electron-rich state of S atoms in the SnS2 catalyst. The finely modulated H* led to a 12-fold decrease in the overpotential of H2O2 generation (H*–OOH*–H2O2–•OH) by decreasing the activation energy barrier of OOH* (rate-determining step). Considering density functional theory calculations, an H*–•OH redox pair suitable for a wide pH range (3–11) was successfully constructed based on the photocathode. The optimal SnS1.85O0.15 AL@TNA photocathode exhibited a ∼90% reduction in Cr(VI) in 10 min and ∼70% TOC removal of 4-nitrophenol, nearly 2- and 3-fold higher than that without oxygen incorporation. Electron spin resonance spectrometry and radical quenching experiments verified that H* and the derived •OH via 1-electron and 3-electron reduction were the main active species. Operando Raman spectroscopy confirmed that the stable SnO2 phase helped constantly activate the production of H* and •OH.
中文翻译:

在 SnS2 原子层中加入氧原子以同时稳定氢原子并加速羟基自由基的产生以进行水净化
光电催化(PEC)是解决各种污染物的有效方法。表面吸附的原子氢 (H*) 和羟基自由基 (•OH) 在 PEC 过程中起关键作用。然而,H* 的不稳定性和•OH 的低产量极大地限制了PEC 效率。在这项研究中,我们注意到加入氧原子可以通过在 SnS 2催化剂中产生局部有利的 S 原子富电子状态来调节 H* 的行为。精细调制的 H* 导致 H 2 O 2生成的过电位(H*–OOH*–H 2 O 2–•OH) 通过降低 OOH* 的活化能垒(速率决定步骤)。考虑到密度泛函理论计算,基于光电阴极成功构建了适用于宽pH范围(3-11)的H*-OH氧化还原对。最佳的 SnS 1.85 O 0.15 AL@TNA 光电阴极在 10 分钟内显示出约 90% 的 Cr(VI) 减少和 约 70% 的 4-硝基苯酚 TOC 去除,比没有加入氧的情况高出近 2 倍和 3 倍。电子自旋共振光谱和自由基猝灭实验证实H*和通过1-电子和3-电子还原衍生的•OH是主要的活性物种。操作拉曼光谱证实,稳定的 SnO 2相有助于不断激活 H* 和•OH 的产生。
更新日期:2022-03-29
中文翻译:

在 SnS2 原子层中加入氧原子以同时稳定氢原子并加速羟基自由基的产生以进行水净化
光电催化(PEC)是解决各种污染物的有效方法。表面吸附的原子氢 (H*) 和羟基自由基 (•OH) 在 PEC 过程中起关键作用。然而,H* 的不稳定性和•OH 的低产量极大地限制了PEC 效率。在这项研究中,我们注意到加入氧原子可以通过在 SnS 2催化剂中产生局部有利的 S 原子富电子状态来调节 H* 的行为。精细调制的 H* 导致 H 2 O 2生成的过电位(H*–OOH*–H 2 O 2–•OH) 通过降低 OOH* 的活化能垒(速率决定步骤)。考虑到密度泛函理论计算,基于光电阴极成功构建了适用于宽pH范围(3-11)的H*-OH氧化还原对。最佳的 SnS 1.85 O 0.15 AL@TNA 光电阴极在 10 分钟内显示出约 90% 的 Cr(VI) 减少和 约 70% 的 4-硝基苯酚 TOC 去除,比没有加入氧的情况高出近 2 倍和 3 倍。电子自旋共振光谱和自由基猝灭实验证实H*和通过1-电子和3-电子还原衍生的•OH是主要的活性物种。操作拉曼光谱证实,稳定的 SnO 2相有助于不断激活 H* 和•OH 的产生。

































 京公网安备 11010802027423号
京公网安备 11010802027423号