Molecular Therapy - Nucleic Acids ( IF 6.5 ) Pub Date : 2022-03-28 , DOI: 10.1016/j.omtn.2022.03.022 Evangelos Konstantinidis 1 , Agnieszka Molisak 1 , Florian Perrin 2 , Linn Streubel-Gallasch 1 , Sarah Fayad 1 , Daniel Y Kim 3, 4, 5 , Karl Petri 3, 4, 5, 6 , Martin J Aryee 3, 4, 6, 7 , Ximena Aguilar 1 , Bence György 8, 9 , Vilmantas Giedraitis 1 , J Keith Joung 3, 4, 5, 6 , Vikram Pattanayak 3, 4, 5, 6 , Magnus Essand 10 , Anna Erlandsson 1 , Oksana Berezovska 2 , Martin Ingelsson 1, 11, 12
|
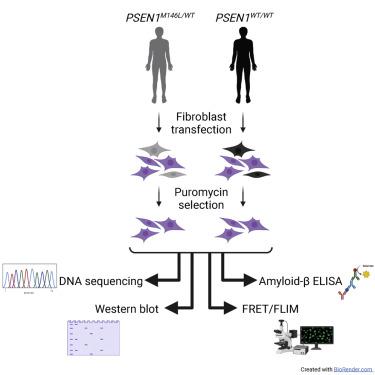
Presenilin 1 (PS1) is a central component of γ-secretase, an enzymatic complex involved in the generation of the amyloid-β (Aβ) peptide that deposits as plaques in the Alzheimer’s disease (AD) brain. The M146L mutation in the PS1 gene (PSEN1) leads to an autosomal dominant form of early-onset AD by promoting a relative increase in the generation of the more aggregation-prone Aβ42. This change is evident not only in the brain but also in peripheral cells of mutation carriers. In this study we used the CRISPR-Cas9 system from Streptococcus pyogenes to selectively disrupt the PSEN1M146L allele in human fibroblasts. A disruption of more than 50% of mutant alleles was observed in all CRISPR-Cas9-treated samples, resulting in reduced extracellular Aβ42/40 ratios. Fluorescence resonance energy transfer-based conformation and western blot analyses indicated that CRISPR-Cas9 treatment also affects the overall PS1 conformation and reduces PS1 levels. Moreover, our guide RNA did not lead to any detectable editing at the highest-ranking candidate off-target sites identified by ONE-seq and CIRCLE-seq. Overall, our data support the effectiveness of CRISPR-Cas9 in selectively targeting the PSEN1M146L allele and counteracting the AD-associated phenotype. We believe that this system could be developed into a therapeutic strategy for patients with this and other dominant mutations leading to early-onset AD.
中文翻译:

CRISPR-Cas9 治疗可部分恢复患有阿尔茨海默病 PSEN1 M146L 突变的人类成纤维细胞中的淀粉样蛋白-β 42/40
早老素 1 (PS1) 是 γ 分泌酶的核心成分,γ 分泌酶是一种酶复合物,参与β淀粉样蛋白 (Aβ) 肽的生成,该肽以斑块形式沉积在阿尔茨海默病 (AD) 大脑中。 PS1 基因 ( PSEN1 ) 中的 M146L 突变通过促进更容易聚集的 Aβ42 生成相对增加,导致常染色体显性形式的早发性 AD。这种变化不仅在大脑中很明显,而且在突变携带者的外周细胞中也很明显。在这项研究中,我们使用化脓性链球菌的 CRISPR-Cas9 系统选择性破坏人类成纤维细胞中的PSEN1 M146L等位基因。在所有 CRISPR-Cas9 处理的样本中观察到超过 50% 的突变等位基因被破坏,导致细胞外 Aβ42/40 比率降低。基于荧光共振能量转移的构象和蛋白质印迹分析表明,CRISPR-Cas9 处理还会影响整体 PS1 构象并降低 PS1 水平。此外,我们的指导 RNA 并未在 ONE-seq 和 CIRCLE-seq 识别的排名最高的候选脱靶位点处导致任何可检测到的编辑。总体而言,我们的数据支持 CRISPR-Cas9 在选择性靶向PSEN1 M146L等位基因并抵消 AD 相关表型方面的有效性。我们相信,该系统可以发展成为一种治疗策略,用于治疗患有这种突变和其他导致早发性 AD 的显性突变的患者。































 京公网安备 11010802027423号
京公网安备 11010802027423号