当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sequence Isomerism in [3]Rotaxanes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2010-04-07 , DOI: 10.1021/ja1006838 Anne-Marie L. Fuller 1 , David A. Leigh 1 , Paul J. Lusby 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2010-04-07 , DOI: 10.1021/ja1006838 Anne-Marie L. Fuller 1 , David A. Leigh 1 , Paul J. Lusby 1
Affiliation
|
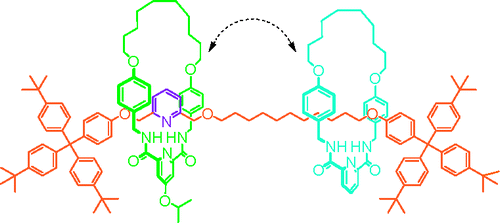
We describe a strategy for assembling different macrocycles onto a nonsymmetrical rotaxane thread in a precise sequence. If the macrocycles are small and rigid enough so that they cannot pass each other then the sequence is maintained mechanically, affording stereoisomerism in a manner reminiscent of atropisomerism. The method is exemplified through the synthesis of a pair of [3]rotaxane diastereomers that are constitutionally identical other than for the sequence of the different macrocycles on the thread. The synthesis features the iterative binding of different palladium(II) pyridine-2,6-dicarboxamide complexes to a pyridine ligand on the thread followed by their macrocyclization by ring-closing olefin metathesis. Removal of the palladium(II) from the first rotaxane formed frees the pyridine site to coordinate to a second, different, palladium(II) pyridine-2,6-dicarboxamide unit which, following macrocyclization, provides a multiring rotaxane of predetermined macrocycle sequence.
中文翻译:

[3]轮烷中的序列异构
我们描述了一种以精确的顺序将不同的大环组装到非对称轮烷线的策略。如果大环足够小且足够刚性,以至于它们不能相互通过,那么序列会机械地保持,以一种让人想起阻转异构的方式提供立体异构。该方法通过合成一对 [3] 轮烷非对映异构体来举例说明,这些非对映异构体除了线上不同大环的序列外在结构上是相同的。该合成的特点是不同的钯 (II) 吡啶-2,6-二甲酰胺复合物与螺纹上的吡啶配体反复结合,然后通过闭环烯烃复分解进行大环化。从形成的第一个轮烷中去除钯 (II) 释放吡啶位点以配位到第二个不同的,
更新日期:2010-04-07
中文翻译:

[3]轮烷中的序列异构
我们描述了一种以精确的顺序将不同的大环组装到非对称轮烷线的策略。如果大环足够小且足够刚性,以至于它们不能相互通过,那么序列会机械地保持,以一种让人想起阻转异构的方式提供立体异构。该方法通过合成一对 [3] 轮烷非对映异构体来举例说明,这些非对映异构体除了线上不同大环的序列外在结构上是相同的。该合成的特点是不同的钯 (II) 吡啶-2,6-二甲酰胺复合物与螺纹上的吡啶配体反复结合,然后通过闭环烯烃复分解进行大环化。从形成的第一个轮烷中去除钯 (II) 释放吡啶位点以配位到第二个不同的,


















































 京公网安备 11010802027423号
京公网安备 11010802027423号