当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rapid Allylic 1,6 H-Atom Transfer in an Unsaturated Criegee Intermediate
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-03-28 , DOI: 10.1021/jacs.2c00055 Anne S Hansen 1 , Yujie Qian 1 , Christopher A Sojdak 1 , Marisa C Kozlowski 1 , Vincent J Esposito 1 , Joseph S Francisco 1 , Stephen J Klippenstein 2 , Marsha I Lester 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-03-28 , DOI: 10.1021/jacs.2c00055 Anne S Hansen 1 , Yujie Qian 1 , Christopher A Sojdak 1 , Marisa C Kozlowski 1 , Vincent J Esposito 1 , Joseph S Francisco 1 , Stephen J Klippenstein 2 , Marsha I Lester 1
Affiliation
|
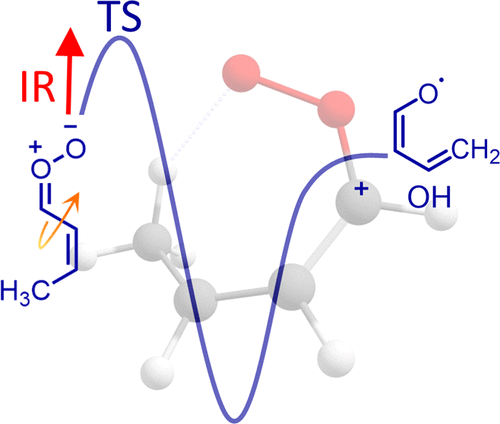
A novel allylic 1,6 hydrogen-atom-transfer mechanism is established through infrared activation of the 2-butenal oxide Criegee intermediate, resulting in very rapid unimolecular decay to hydroxyl (OH) radical products. A new precursor, Z/E-1,3-diiodobut-1-ene, is synthesized and photolyzed in the presence of oxygen to generate a new four-carbon Criegee intermediate with extended conjugation across the vinyl and carbonyl oxide groups that facilitates rapid allylic 1,6 H-atom transfer. A low-energy reaction pathway involving isomerization of 2-butenal oxide from a lower-energy (tZZ) conformer to a higher-energy (cZZ) conformer followed by 1,6 hydrogen transfer via a seven-membered ring transition state is predicted theoretically and shown experimentally to yield OH products. The low-lying (tZZ) conformer of 2-butenal oxide is identified based on computed anharmonic frequencies and intensities of its conformers. Experimental IR action spectra recorded in the fundamental CH stretch region with OH product detection by UV laser-induced fluorescence reveal a distinctive IR transition of the low-lying (tZZ) conformer at 2996 cm–1 that results in rapid unimolecular decay to OH products. Statistical RRKM calculations involving a combination of conformational isomerization and unimolecular decay via 1,6 H-transfer yield an effective decay rate keff(E) on the order of 108 s–1 at ca. 3000 cm–1 in good accord with the experiment. Unimolecular decay proceeds with significant enhancement due to quantum mechanical tunneling. A rapid thermal decay rate of ca. 106 s–1 is predicted by master-equation modeling of 2-butenal oxide at 298 K, 1 bar. This novel unimolecular decay pathway is expected to increase the nonphotolytic production of OH radicals upon alkene ozonolysis in the troposphere.
中文翻译:

不饱和 Criegee 中间体中的快速烯丙基 1,6 H 原子转移
通过红外活化 2-丁烯氧化物 Criegee 中间体,建立了一种新的烯丙基 1,6 氢原子转移机制,导致非常快速的单分子衰变为羟基 (OH) 自由基产物。一种新的前体Z / E -1,3-diiodobut-1-ene 被合成并在氧气存在下光解以产生新的四碳 Criegee 中间体,该中间体在乙烯基和羰基氧化物基团之间具有扩展共轭,有助于快速烯丙基1,6 H 原子转移。一种低能反应途径,涉及将 2-氧化丁烯醛从低能 ( tZZ ) 构象异构体转化为高能 ( cZZ ) 构象异构体,然后通过1,6 氢转移理论上预测了一个七元环过渡态,并在实验上表明会产生 OH 产物。2-氧化丁烯醛的低位 ( tZZ ) 构象异构体是根据计算的非谐波频率和其构象异构体的强度来识别的。用紫外激光诱导荧光检测 OH 产物在基本 CH 拉伸区域记录的实验 IR 作用光谱揭示了低位 ( tZZ ) 构象异构体在 2996 cm –1处的独特 IR 转变,导致单分子快速衰减为 OH 产物。统计 RRKM 计算涉及构象异构化和通过1,6 H-转移的单分子衰变的组合产生有效的衰变率k eff ( E) 大约 10 8 s –1在 ca。3000 cm -1与实验吻合较好。由于量子力学隧道效应,单分子衰变显着增强。约的快速热衰减率。10 6 s –1通过 2-丁烯氧化物的主方程模型在 298 K、1 bar 下预测。这种新颖的单分子衰变途径有望增加对流层中烯烃臭氧分解时 OH 自由基的非光解产生。
更新日期:2022-03-28
中文翻译:

不饱和 Criegee 中间体中的快速烯丙基 1,6 H 原子转移
通过红外活化 2-丁烯氧化物 Criegee 中间体,建立了一种新的烯丙基 1,6 氢原子转移机制,导致非常快速的单分子衰变为羟基 (OH) 自由基产物。一种新的前体Z / E -1,3-diiodobut-1-ene 被合成并在氧气存在下光解以产生新的四碳 Criegee 中间体,该中间体在乙烯基和羰基氧化物基团之间具有扩展共轭,有助于快速烯丙基1,6 H 原子转移。一种低能反应途径,涉及将 2-氧化丁烯醛从低能 ( tZZ ) 构象异构体转化为高能 ( cZZ ) 构象异构体,然后通过1,6 氢转移理论上预测了一个七元环过渡态,并在实验上表明会产生 OH 产物。2-氧化丁烯醛的低位 ( tZZ ) 构象异构体是根据计算的非谐波频率和其构象异构体的强度来识别的。用紫外激光诱导荧光检测 OH 产物在基本 CH 拉伸区域记录的实验 IR 作用光谱揭示了低位 ( tZZ ) 构象异构体在 2996 cm –1处的独特 IR 转变,导致单分子快速衰减为 OH 产物。统计 RRKM 计算涉及构象异构化和通过1,6 H-转移的单分子衰变的组合产生有效的衰变率k eff ( E) 大约 10 8 s –1在 ca。3000 cm -1与实验吻合较好。由于量子力学隧道效应,单分子衰变显着增强。约的快速热衰减率。10 6 s –1通过 2-丁烯氧化物的主方程模型在 298 K、1 bar 下预测。这种新颖的单分子衰变途径有望增加对流层中烯烃臭氧分解时 OH 自由基的非光解产生。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号