当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Amine Organocatalysis of Remote, Chemoselective C(sp3)–H Hydroxylation
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-03-28 , DOI: 10.1021/acscatal.2c00392
Philip L Hahn 1 , Jared M Lowe 1 , Yubo Xu 1 , Kevin L Burns 1 , Michael K Hilinski 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-03-28 , DOI: 10.1021/acscatal.2c00392
Philip L Hahn 1 , Jared M Lowe 1 , Yubo Xu 1 , Kevin L Burns 1 , Michael K Hilinski 1
Affiliation
|
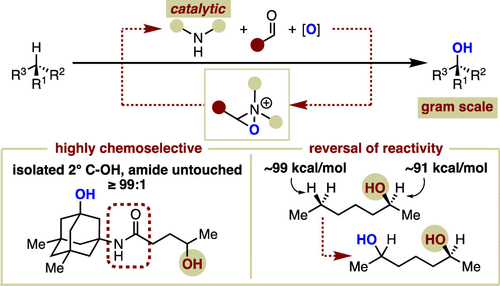
We introduce an organocatalytic approach for oxaziridinium-mediated C–H hydroxylation that employs secondary amines as catalysts. We also demonstrate the advantages of this operationally simple catalytic strategy for achieving high yielding and highly selective remote hydroxylation of compounds bearing oxidation-sensitive functional groups such as alcohols, ethers, carbamates, and amides. By employing hexafluoroisopropanol as the solvent in the absence of water, a proposed hydrogen-bonding effect leads to, among other advantages, as high as ≥99:1 chemoselectivity for remote aliphatic hydroxylation of 2° alcohols, an otherwise unsolved synthetic challenge normally complicated by substantial amounts of alcohol oxidation. Initial studies of the reaction mechanism indicate the formation of an oxaziridinium salt as the active oxidant and a C–H oxidation step that proceeds in a stereospecific manner via concerted insertion or hydrogen atom-transfer/radical rebound. Furthermore, preliminary results indicate that site selectivity can be affected by amine catalyst structure.
中文翻译:

远程化学选择性 C(sp3)–H 羟基化的胺有机催化
我们介绍了一种使用仲胺作为催化剂的恶氮丙啶介导的 C-H 羟基化的有机催化方法。我们还展示了这种操作简单的催化策略在实现带有氧化敏感官能团(如醇、醚、氨基甲酸酯和酰胺)的化合物的高产率和高选择性远程羟基化方面的优势。通过在不存在水的情况下使用六氟异丙醇作为溶剂,所提出的氢键效应除其他优势外,对 2° 醇的远程脂肪族羟基化具有高达 ≥ 99:1 的化学选择性,这是一个未解决的合成挑战,通常复杂化大量的酒精氧化。反应机制的初步研究表明,氧氮丙啶盐作为活性氧化剂的形成和 C-H 氧化步骤通过协同插入或氢原子转移/自由基反弹以立体有择的方式进行。此外,初步结果表明位点选择性会受到胺催化剂结构的影响。
更新日期:2022-03-28
中文翻译:

远程化学选择性 C(sp3)–H 羟基化的胺有机催化
我们介绍了一种使用仲胺作为催化剂的恶氮丙啶介导的 C-H 羟基化的有机催化方法。我们还展示了这种操作简单的催化策略在实现带有氧化敏感官能团(如醇、醚、氨基甲酸酯和酰胺)的化合物的高产率和高选择性远程羟基化方面的优势。通过在不存在水的情况下使用六氟异丙醇作为溶剂,所提出的氢键效应除其他优势外,对 2° 醇的远程脂肪族羟基化具有高达 ≥ 99:1 的化学选择性,这是一个未解决的合成挑战,通常复杂化大量的酒精氧化。反应机制的初步研究表明,氧氮丙啶盐作为活性氧化剂的形成和 C-H 氧化步骤通过协同插入或氢原子转移/自由基反弹以立体有择的方式进行。此外,初步结果表明位点选择性会受到胺催化剂结构的影响。

































 京公网安备 11010802027423号
京公网安备 11010802027423号