当前位置:
X-MOL 学术
›
ACS Environ. Au
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
PIMT-Mediated Labeling of l-Isoaspartic Acid with Tris Facilitates Identification of Isomerization Sites in Long-Lived Proteins
ACS Environmental Au ( IF 6.7 ) Pub Date : 2022-02-03 , DOI: 10.1021/jasms.1c00355 Jacob W. Silzel 1 , Tyler R. Lambeth 1 , Ryan R. Julian 1
ACS Environmental Au ( IF 6.7 ) Pub Date : 2022-02-03 , DOI: 10.1021/jasms.1c00355 Jacob W. Silzel 1 , Tyler R. Lambeth 1 , Ryan R. Julian 1
Affiliation
|
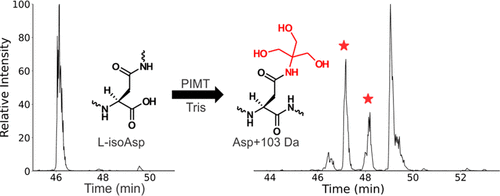
Isomerization of individual residues in long-lived proteins (LLPs) is a subject of growing interest in connection with many age-related human diseases. When isomerization occurs in LLPs, it can lead to deleterious changes in protein structure, function, and proteolytic degradation. Herein, we present a novel labeling technique for rapid identification of l-isoAsp using the enzyme protein l-isoaspartyl methyltransferase (PIMT) and Tris. The succinimide intermediate formed during reaction of l-isoAsp-containing peptides with PIMT and S-adenosyl methionine (SAM) is reactive with Tris base and results in a Tris-modified aspartic acid residue with a mass shift of +103 Da. Tris-modified aspartic acid exhibits prominent and repeated neutral loss of water when subjected to collisional activation. In addition, another dissociation pathway regenerates the original peptide following loss of a characteristic mass shift. Furthermore, it is demonstrated that Tris modification can be used to identify sites of isomerization in LLPs from biological samples such as the lens of the eye. This approach simplifies identification by labeling isomerization sites with a tag that causes a mass shift and provides characteristic loss during collisional activation.
中文翻译:

PIMT 介导的用 Tris 标记 l-异天冬氨酸有助于鉴定长寿命蛋白质中的异构化位点
长寿命蛋白质 (LLP) 中单个残基的异构化是与许多与年龄有关的人类疾病相关的越来越感兴趣的主题。当 LLP 中发生异构化时,会导致蛋白质结构、功能和蛋白水解降解的有害变化。在此,我们提出了一种使用酶蛋白l-异天冬氨酰甲基转移酶 (PIMT) 和 Tris快速识别l- isoAsp的新型标记技术。在含有l -isoAsp 的肽与 PIMT 和S反应过程中形成的琥珀酰亚胺中间体-腺苷甲硫氨酸 (SAM) 可与 Tris 碱反应并产生具有 +103 Da 质量位移的 Tris 修饰的天冬氨酸残基。当受到碰撞活化时,Tris 修饰的天冬氨酸表现出显着且重复的中性失水。此外,另一种解离途径在失去特征质量转移后再生原始肽。此外,已证明 Tris 修饰可用于识别来自生物样品(例如眼睛晶状体)的 LLP 中的异构化位点。这种方法通过用标签标记异构化位点来简化识别,该标签会导致质量转移并在碰撞激活期间提供特征损失。
更新日期:2022-02-03
中文翻译:

PIMT 介导的用 Tris 标记 l-异天冬氨酸有助于鉴定长寿命蛋白质中的异构化位点
长寿命蛋白质 (LLP) 中单个残基的异构化是与许多与年龄有关的人类疾病相关的越来越感兴趣的主题。当 LLP 中发生异构化时,会导致蛋白质结构、功能和蛋白水解降解的有害变化。在此,我们提出了一种使用酶蛋白l-异天冬氨酰甲基转移酶 (PIMT) 和 Tris快速识别l- isoAsp的新型标记技术。在含有l -isoAsp 的肽与 PIMT 和S反应过程中形成的琥珀酰亚胺中间体-腺苷甲硫氨酸 (SAM) 可与 Tris 碱反应并产生具有 +103 Da 质量位移的 Tris 修饰的天冬氨酸残基。当受到碰撞活化时,Tris 修饰的天冬氨酸表现出显着且重复的中性失水。此外,另一种解离途径在失去特征质量转移后再生原始肽。此外,已证明 Tris 修饰可用于识别来自生物样品(例如眼睛晶状体)的 LLP 中的异构化位点。这种方法通过用标签标记异构化位点来简化识别,该标签会导致质量转移并在碰撞激活期间提供特征损失。






























 京公网安备 11010802027423号
京公网安备 11010802027423号