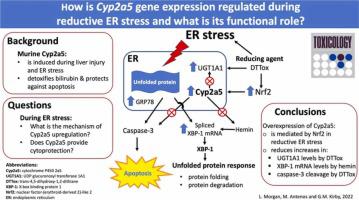
细胞色素 P450 2a5 (Cyp2a5) 与其他 P450 酶不同,它是在对肝脏有害的条件下在小鼠肝细胞的内质网 (ER) 中诱导产生的。如果不加以纠正,这些情况会导致内质网应激,最终导致细胞凋亡。我们之前表明,小鼠肝脏 Cyp2a5 在二硫苏糖醇分子内二硫键反式-4,5-二羟基-1,2-二噻烷 (DTT ox ) 引起的还原性内质网应激过程中被诱导,并且 Cyp2a5 的过度表达提供了针对细胞凋亡的部分保护由于胆红素 (BR),一种已知会导致 ER 应激的化合物。本研究的目的是研究DTTox对Cyp2a5基因的调控机制,并确定Cyp2a5在还原性内质网应激期间是否发挥细胞保护作用。暴露于 DTT ox (10 mM) 和另一种还原性 ER 应激源 2-巯基乙醇 (1 mM) 48 小时,原代小鼠肝细胞中的 Cyp2a5 蛋白水平显着增加。此外,DTT ox通过涉及转录因子核因子(红细胞衍生 2)样 2 (Nrf2) 的机制反式激活Cyp2a5 。BR 结合酶、UDP 葡萄糖醛酸基转移酶 1A1 (UGT1A1) 的表达在 DTT ox处理后也有所增加,但由于 Cyp2a5 过表达而降低。高铁血红素 (Hemin) 是 Cyp2a5 的卟啉诱导剂,可诱导 X-box 结合蛋白 1 (XBP-1) 的 mRNA 剪接,X-box 结合蛋白 1 (XBP-1) 是一种参与 ER 应激反应的转录因子,然而,Cyp2a5 过表达也会降低这种剪接。最后,Cyp2a5 的过表达部分阻断了Hepa 1-6 细胞中DTT ox介导的 caspase-3 裂解,表明在内质网应激期间具有细胞保护作用。这些发现表明,在减少 ER 的环境中,Nrf2 介导的 Cyp2a5 诱导通过减少 XBP-1 mRNA 剪接和 caspase-3 切割,提供了针对 ER 应激诱导的细胞凋亡的部分保护。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Nrf2-mediated induction of Cyp2a5 partially protects against reductive endoplasmic reticulum stress in mouse hepatocytes
Cytochrome P450 2a5 (Cyp2a5) is distinct from other P450 enzymes in that it is induced in the endoplasmic reticulum (ER) of mouse hepatocytes in conditions that are injurious to the liver. These conditions cause ER stress eventually resulting in apoptosis if not rectified. We previously showed that mouse hepatic Cyp2a5 is induced during reductive ER stress caused by the intramolecular disulfide form of dithiothreitol, trans-4,5-dihydroxy-1,2-dithiane (DTTox), and that overexpression of Cyp2a5 provides partial protection against apoptosis due to bilirubin (BR), a compound known to cause ER stress. The purpose of this study was to investigate the mechanism of Cyp2a5 gene regulation by DTTox and to determine if Cyp2a5 plays a cytoprotective role during reductive ER stress. Exposure to DTTox (10 mM) and another reductive ER stressor, 2-mercaptoethanol (1 mM), for 48 h markedly increased Cyp2a5 protein levels in primary mouse hepatocytes. In addition, DTTox transactivated Cyp2a5 via a mechanism involving the transcription factor nuclear factor-(erythroid-derived 2)-like 2 (Nrf2). Expression of the BR-conjugating enzyme, UDP glucuronosyl transferase 1A1 (UGT1A1) was also increased after DTTox treatment, however, this was reduced by Cyp2a5 overexpression. Hemin, a porphyrin inducer of Cyp2a5, induced mRNA splicing of X-box binding protein 1 (XBP-1), a transcription factor involved in the ER stress response, however, this was also reduced by Cyp2a5 overexpression. Finally, overexpression of Cyp2a5 partially blocked DTTox-mediated caspase-3 cleavage in Hepa 1–6 cells suggesting a cytoprotective role during ER stress. These findings demonstrate that Nrf2-mediated induction of Cyp2a5 in a reducing ER environment provides partial protection against ER stress-induced apoptosis by decreasing XBP-1 mRNA splicing and caspase-3 cleavage.


































 京公网安备 11010802027423号
京公网安备 11010802027423号