当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-Based Discovery and Optimization of Furo[3,2-c]pyridin-4(5H)-one Derivatives as Potent and Second Bromodomain (BD2)-Selective Bromo and Extra Terminal Domain (BET) Inhibitors
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2022-03-25 , DOI: 10.1021/acs.jmedchem.2c00100 Junhua Li 1, 2 , Cheng Zhang 1 , Hongrui Xu 1, 3 , Chao Wang 1, 2 , Ruibo Dong 1, 4 , Hui Shen 1, 2 , Xiaoxi Zhuang 1 , Xiaoshan Chen 1, 2 , Qiu Li 1, 2 , Jibu Lu 1, 2 , Maofeng Zhang 5 , Xishan Wu 1 , Kerry M Loomes 6 , Yulai Zhou 4 , Yan Zhang 1 , Jinsong Liu 1, 7 , Yong Xu 1, 3, 7, 8
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2022-03-25 , DOI: 10.1021/acs.jmedchem.2c00100 Junhua Li 1, 2 , Cheng Zhang 1 , Hongrui Xu 1, 3 , Chao Wang 1, 2 , Ruibo Dong 1, 4 , Hui Shen 1, 2 , Xiaoxi Zhuang 1 , Xiaoshan Chen 1, 2 , Qiu Li 1, 2 , Jibu Lu 1, 2 , Maofeng Zhang 5 , Xishan Wu 1 , Kerry M Loomes 6 , Yulai Zhou 4 , Yan Zhang 1 , Jinsong Liu 1, 7 , Yong Xu 1, 3, 7, 8
Affiliation
|
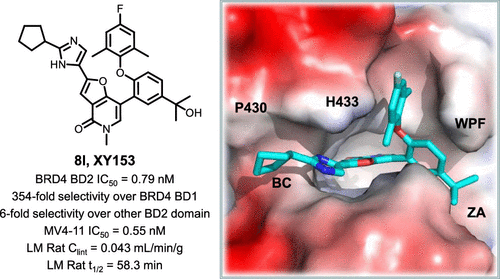
Pan-bromodomain and extra terminal (Pan-BET) inhibitors show profound efficacy but exhibit pharmacology-driven toxicities in clinical trials. The development of domain-selective BET inhibitors to separate efficacy and toxicity is urgently needed. Herein, we report a series of furo[3,2-c]pyridin-4(5H)-one derivatives as novel BD2-selective BET inhibitors. The representative compound 8l (XY153) potently bound to BRD4 BD2 with an half-maximum inhibitory concentration (IC50) value of 0.79 nM and displayed 354-fold selectivity over BRD4 BD1. Besides, 8l exhibited 6-fold BRD4 BD2 domain selectivity over other BET BD2 domains. Compound 8l displayed potent antiproliferative activity against multiple tumor cell lines, especially MV4-11 (IC50 = 0.55 nM), while showing weak cytotoxicity against the normal lung fibroblast cell line. It highlights the safety profile of this series of BD2 inhibitors. 8l also demonstrated good metabolic stability in vitro. These data indicate that 8l may serve as a new and valuable lead compound for the development of potential therapeutics against acute myeloid leukemia (AML).
中文翻译:

基于结构的 Furo[3,2-c]pyridin-4(5H)-one 衍生物作为有效和第二溴结构域 (BD2)-选择性溴和额外末端结构域 (BET) 抑制剂的发现和优化
泛溴结构域和额外末端 (Pan-BET) 抑制剂显示出深远的疗效,但在临床试验中表现出药理学驱动的毒性。迫切需要开发域选择性 BET 抑制剂来区分疗效和毒性。在此,我们报告了一系列作为新型 BD2 选择性 BET 抑制剂的 furo[3,2 - c ]pyridin-4(5 H )-one 衍生物。代表性化合物8l (XY153) 与 BRD4 BD2 有效结合,半数最大抑制浓度 (IC 50 ) 值为 0.79 nM,并显示出比 BRD4 BD1 高 354 倍的选择性。此外,8l表现出比其他 BET BD2 结构域高 6 倍的 BRD4 BD2 结构域选择性。化合物8l对多种肿瘤细胞系显示出有效的抗增殖活性,尤其是 MV4-11 (IC 50 = 0.55 nM),同时对正常肺成纤维细胞系显示出较弱的细胞毒性。它突出了这一系列 BD2 抑制剂的安全性。8l还表现出良好的体外代谢稳定性。这些数据表明,8l可作为一种新的有价值的先导化合物,用于开发针对急性髓性白血病 (AML) 的潜在疗法。
更新日期:2022-03-25
中文翻译:

基于结构的 Furo[3,2-c]pyridin-4(5H)-one 衍生物作为有效和第二溴结构域 (BD2)-选择性溴和额外末端结构域 (BET) 抑制剂的发现和优化
泛溴结构域和额外末端 (Pan-BET) 抑制剂显示出深远的疗效,但在临床试验中表现出药理学驱动的毒性。迫切需要开发域选择性 BET 抑制剂来区分疗效和毒性。在此,我们报告了一系列作为新型 BD2 选择性 BET 抑制剂的 furo[3,2 - c ]pyridin-4(5 H )-one 衍生物。代表性化合物8l (XY153) 与 BRD4 BD2 有效结合,半数最大抑制浓度 (IC 50 ) 值为 0.79 nM,并显示出比 BRD4 BD1 高 354 倍的选择性。此外,8l表现出比其他 BET BD2 结构域高 6 倍的 BRD4 BD2 结构域选择性。化合物8l对多种肿瘤细胞系显示出有效的抗增殖活性,尤其是 MV4-11 (IC 50 = 0.55 nM),同时对正常肺成纤维细胞系显示出较弱的细胞毒性。它突出了这一系列 BD2 抑制剂的安全性。8l还表现出良好的体外代谢稳定性。这些数据表明,8l可作为一种新的有价值的先导化合物,用于开发针对急性髓性白血病 (AML) 的潜在疗法。






























 京公网安备 11010802027423号
京公网安备 11010802027423号