Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2022-03-26 , DOI: 10.1016/j.apcatb.2022.121346 Yuanzheng Zhang 1 , Xiang Chen 1 , Weilai Wang 1 , Lifeng Yin 1 , John C. Crittenden 1, 2
|
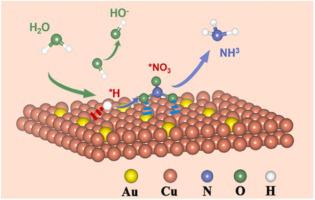
Electrocatalytic reduction of nitrate (NO3–) to ammonia (NH3) in wastewater is a promising economic process for NH3 synthesis. This work designed and prepared Au1Cu (111) single-atom alloys with surface Cu vacancies (VCu-Au1Cu SAAs), which exhibited superior NH3 Faradaic efficiency (98.7%) with a production rate of 555 μg h–1 cm–2 at −0.2 V vs. RHE, while negligible activity decay was found after a durability test. Meanwhile, 97% of produced NH3 can be recovered by a simple membrane distillation. Characterizations evidence that electron migration from Cu to Au atoms creates electron-deficient Cu active sites in VCu-Au1Cu SAAs, which promote the generation of active hydrogen species (*H) that can readily hydrogenate NO3–. Theoretical calculation reveals that the bi-functional Cu sites not only promote the activation of water to produce *H but also lower the energy barrier of *NH3 desorption from the catalyst surface.
中文翻译:

有缺陷的 Au1Cu (111) 单原子合金电催化硝酸盐还原成氨
将废水中的硝酸盐(NO 3 –)电催化还原为氨(NH 3)是一种很有前景的经济合成NH 3工艺。本工作设计并制备了具有表面Cu空位(V Cu -Au 1 Cu SAA)的Au 1 Cu (111)单原子合金,其NH 3法拉第效率(98.7%)具有555 μg h -1的生产率。cm –2 at -0.2 V vs. RHE,而在耐久性测试后发现活性衰减可忽略不计。同时,产生的 NH 3的 97%可以通过简单的膜蒸馏回收。表征证据表明,电子从 Cu 到 Au 原子的迁移在 V Cu -Au 1 Cu SAA 中产生了缺电子的 Cu 活性位点,这促进了活性氢物质 (*H) 的产生,可以很容易地氢化 NO 3 –。理论计算表明,双功能Cu位点不仅促进了水活化生成*H,而且降低了*NH 3从催化剂表面解吸的能垒。































 京公网安备 11010802027423号
京公网安备 11010802027423号