当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Access to Cyclic N-Trifluoromethyl Ureas through Photocatalytic Activation of Carbamoyl Azides
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-03-25 , DOI: 10.1021/jacs.2c02004 Samir Bouayad-Gervais 1 , Christian D-T Nielsen 1 , Abdurrahman Turksoy 1 , Theresa Sperger 1 , Kristina Deckers 1 , Franziska Schoenebeck 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-03-25 , DOI: 10.1021/jacs.2c02004 Samir Bouayad-Gervais 1 , Christian D-T Nielsen 1 , Abdurrahman Turksoy 1 , Theresa Sperger 1 , Kristina Deckers 1 , Franziska Schoenebeck 1
Affiliation
|
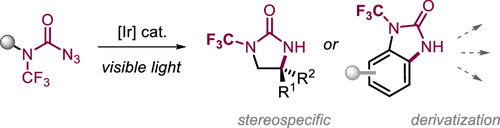
We report the mild activation of carbamoyl azides to the corresponding nitrenes using a blue light/[Ir]-catalyzed strategy, which enables stereospecific access to N-trifluoromethyl imidazolidinones and benzimidazolones. These novel structural motifs proved to be highly robust, allowing their downstream diversification. On the basis of our combined computational and experimental studies, we propose that an electron rebound with the excited metal catalyst is undergone, involving a reduction-triggered nitrogen loss, followed by oxidation to the corresponding carbamoyl nitrene and subsequent C–H insertion.
中文翻译:

通过氨基甲酰叠氮化物的光催化活化获得环状 N-三氟甲基脲
我们报告了使用蓝光/[Ir] 催化的策略将氨基甲酰基叠氮化物温和活化为相应的氮烯,这使得立体定向获得N-三氟甲基咪唑啉酮和苯并咪唑酮成为可能。这些新颖的结构基序被证明是高度稳健的,允许它们的下游多样化。在我们结合计算和实验研究的基础上,我们建议经历电子与激发的金属催化剂的反弹,包括还原引发的氮损失,然后氧化成相应的氨基甲酰基氮烯和随后的 C-H 插入。
更新日期:2022-03-25
中文翻译:

通过氨基甲酰叠氮化物的光催化活化获得环状 N-三氟甲基脲
我们报告了使用蓝光/[Ir] 催化的策略将氨基甲酰基叠氮化物温和活化为相应的氮烯,这使得立体定向获得N-三氟甲基咪唑啉酮和苯并咪唑酮成为可能。这些新颖的结构基序被证明是高度稳健的,允许它们的下游多样化。在我们结合计算和实验研究的基础上,我们建议经历电子与激发的金属催化剂的反弹,包括还原引发的氮损失,然后氧化成相应的氨基甲酰基氮烯和随后的 C-H 插入。































 京公网安备 11010802027423号
京公网安备 11010802027423号