Chemical Physics Letters ( IF 2.8 ) Pub Date : 2022-03-24 , DOI: 10.1016/j.cplett.2022.139581 Tuba Dedecan 1 , Nilay Baylan 1 , İsmail İnci 1
|
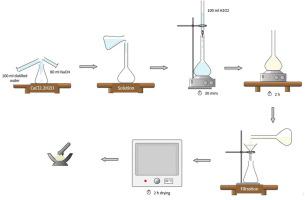
This study aims to propose a novel adsorbent for removal of malic acid from aqueous solutions. In this context, calcium peroxide (CaO2) nanoparticles as novel adsorbent were synthesized, characterized, and assessed the efficiency of in malic acid removal. Followed this, kinetic, thermodynamic, and equilibrium studies were conducted. The average particle diameter and surface area of CaO2 nanoparticles have been found to be 34.77 nm and 13.58 m2.g−1, respectively. The maximum adsorption capacity of CaO2 nanoparticles for malic acid has been determined as 596.86 mg.g−1. The results showed that CaO2 nanoparticles are effective adsorbent in removing malic acid from aqueous solutions.
中文翻译:

过氧化钙纳米颗粒作为去除水溶液中苹果酸的新型吸附剂的合成、表征及应用
本研究旨在提出一种用于从水溶液中去除苹果酸的新型吸附剂。在此背景下,合成、表征和评估了作为新型吸附剂的过氧化钙 (CaO 2 ) 纳米颗粒去除苹果酸的效率。在此之后,进行了动力学、热力学和平衡研究。已发现CaO 2纳米颗粒的平均粒径和表面积分别为 34.77 nm 和 13.58 m 2 .g -1。CaO 2纳米颗粒对苹果酸的最大吸附容量已确定为596.86 mg.g -1。结果表明,CaO 2纳米颗粒是从水溶液中去除苹果酸的有效吸附剂。

































 京公网安备 11010802027423号
京公网安备 11010802027423号