当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sense-and-Respond Payload Delivery Using a Novel Antigen-Inducible Promoter Improves Suboptimal CAR-T Activation
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2022-03-22 , DOI: 10.1021/acssynbio.1c00236 Tingxi Guo 1 , Dacheng Ma 1 , Timothy K Lu 1, 2, 3, 4
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2022-03-22 , DOI: 10.1021/acssynbio.1c00236 Tingxi Guo 1 , Dacheng Ma 1 , Timothy K Lu 1, 2, 3, 4
Affiliation
|
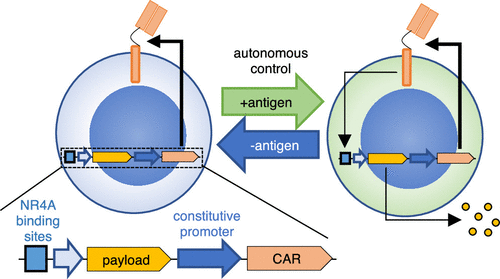
Chimeric antigen receptor (CAR)-T cell therapies demonstrate the clinical potential of lymphocytes engineered with synthetic properties. However, CAR-T cells are ineffective in most solid tumors, partly due to inadequate activation of the infused lymphocytes at the site of malignancy. To selectively enhance antitumor efficacy without exacerbating off-target toxicities, CAR-T cells can be engineered to preferentially deliver immunostimulatory payloads in tumors. Here, we report a novel antigen-inducible promoter for conditional payload expression in primary human T cells. In therapeutic T cell models, the novel NR4A-based promoter induced higher reporter gene expression than the conventional NFAT-based promoter under weakly immunogenic conditions, where payload expression is most needed. Minimal activity was detected from the inducible promoters in the absence of antigen and after withdrawal of stimulation. As a functional proof-of-concept, we used the NR4A-based promoter to express cytokines in an antimesothelin CAR-T model with suboptimal stimulation and observed improved proliferation compared to T cells engineered with the conventional NFAT promoter or CAR alone. Our system achieves CAR-directed payload expression under weakly immunogenic conditions and could enable the next generation of cell therapies with enhanced antitumor efficacy.
中文翻译:

使用新型抗原诱导启动子的感知和响应有效载荷传递改善了次优的 CAR-T 激活
嵌合抗原受体 (CAR)-T 细胞疗法证明了具有合成特性的淋巴细胞的临床潜力。然而,CAR-T 细胞在大多数实体瘤中无效,部分原因是恶性肿瘤部位注入的淋巴细胞激活不足。为了在不加剧脱靶毒性的情况下选择性地增强抗肿瘤功效,可以对 CAR-T 细胞进行工程改造,以优先在肿瘤中递送免疫刺激有效载荷。在这里,我们报告了一种新的抗原诱导启动子,用于在原代人类 T 细胞中进行条件有效载荷表达。在治疗性 T 细胞模型中,在最需要有效载荷表达的弱免疫原性条件下,新型基于 NR4A 的启动子比传统的基于 NFAT 的启动子诱导更高的报告基因表达。在没有抗原和刺激停止后,从诱导型启动子中检测到最小的活性。作为一个功能性的概念验证,我们使用基于 NR4A 的启动子在具有次优刺激的抗间皮素 CAR-T 模型中表达细胞因子,并观察到与使用传统 NFAT 启动子或单独 CAR 改造的 T 细胞相比,增殖得到改善。我们的系统在弱免疫原性条件下实现了 CAR 导向的有效载荷表达,并且可以使下一代细胞疗法具有增强的抗肿瘤功效。我们使用基于 NR4A 的启动子在具有次优刺激的抗肌皮素 CAR-T 模型中表达细胞因子,并观察到与使用常规 NFAT 启动子或单独 CAR 改造的 T 细胞相比,增殖得到改善。我们的系统在弱免疫原性条件下实现了 CAR 导向的有效载荷表达,并且可以使下一代细胞疗法具有增强的抗肿瘤功效。我们使用基于 NR4A 的启动子在具有次优刺激的抗肌皮素 CAR-T 模型中表达细胞因子,并观察到与使用常规 NFAT 启动子或单独 CAR 改造的 T 细胞相比,增殖得到改善。我们的系统在弱免疫原性条件下实现了 CAR 导向的有效载荷表达,并且可以使下一代细胞疗法具有增强的抗肿瘤功效。
更新日期:2022-03-22
中文翻译:

使用新型抗原诱导启动子的感知和响应有效载荷传递改善了次优的 CAR-T 激活
嵌合抗原受体 (CAR)-T 细胞疗法证明了具有合成特性的淋巴细胞的临床潜力。然而,CAR-T 细胞在大多数实体瘤中无效,部分原因是恶性肿瘤部位注入的淋巴细胞激活不足。为了在不加剧脱靶毒性的情况下选择性地增强抗肿瘤功效,可以对 CAR-T 细胞进行工程改造,以优先在肿瘤中递送免疫刺激有效载荷。在这里,我们报告了一种新的抗原诱导启动子,用于在原代人类 T 细胞中进行条件有效载荷表达。在治疗性 T 细胞模型中,在最需要有效载荷表达的弱免疫原性条件下,新型基于 NR4A 的启动子比传统的基于 NFAT 的启动子诱导更高的报告基因表达。在没有抗原和刺激停止后,从诱导型启动子中检测到最小的活性。作为一个功能性的概念验证,我们使用基于 NR4A 的启动子在具有次优刺激的抗间皮素 CAR-T 模型中表达细胞因子,并观察到与使用传统 NFAT 启动子或单独 CAR 改造的 T 细胞相比,增殖得到改善。我们的系统在弱免疫原性条件下实现了 CAR 导向的有效载荷表达,并且可以使下一代细胞疗法具有增强的抗肿瘤功效。我们使用基于 NR4A 的启动子在具有次优刺激的抗肌皮素 CAR-T 模型中表达细胞因子,并观察到与使用常规 NFAT 启动子或单独 CAR 改造的 T 细胞相比,增殖得到改善。我们的系统在弱免疫原性条件下实现了 CAR 导向的有效载荷表达,并且可以使下一代细胞疗法具有增强的抗肿瘤功效。我们使用基于 NR4A 的启动子在具有次优刺激的抗肌皮素 CAR-T 模型中表达细胞因子,并观察到与使用常规 NFAT 启动子或单独 CAR 改造的 T 细胞相比,增殖得到改善。我们的系统在弱免疫原性条件下实现了 CAR 导向的有效载荷表达,并且可以使下一代细胞疗法具有增强的抗肿瘤功效。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号