Separation and Purification Technology ( IF 8.1 ) Pub Date : 2022-03-22 , DOI: 10.1016/j.seppur.2022.120904 Jianhui Xu 1 , Yufeng Liu 1 , Dan Li 1 , Lei Li 1 , Yunfei Zhang 1 , Shenggui Chen 2 , Qi Wu 1 , Pengxu Wang 1 , Chunhui Zhang 1 , Jieyi Sun 1
|
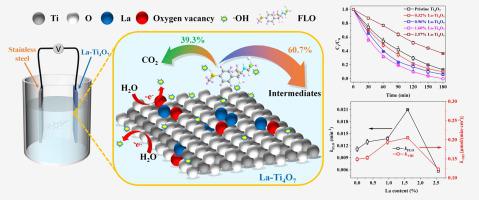
In this study, a novel active La-doped Ti4O7 (La-Ti4O7) electrode was fabricated through a simple one-step spark plasma sintering method. Characterization results revealed that La was successfully incorporated into the crystal lattice of Ti4O7, leading to an increase in the surface oxygen vacancy content (from 26% to 31%), oxygen evolution potential (from 2.24 to 2.75 V vs SCE), hydroxyl radical yield [•OH, from 0.123 to 0.205 μmol/(min·cm2)] and interfacial charge-transfer rate compared to pristine Ti4O7. La-Ti4O7 electrodes achieved efficient anodic oxidation of florfenicol (FLO, one of the most widely used antibiotics), which mainly due to the indirect oxidation mediated by electro-generated •OH. The degradation of FLO by La-Ti4O7 electrodes fitted well with the pseudo-first-order kinetic model, and the optimal degradation rate constant (kFLO, 0.021 min−1) was achieved by 1.60% La-Ti4O7 electrode. In addition, the degradation efficiency of FLO increased with the increasing current density, decreasing pH and co-existing Cl-, while an opposite pattern was observed with co-existing NO3–. Seven degradation products of FLO were identified by UPLC-MS/MS. The main degradation pathways included hydrolysis, hydroxylation, dechlorination and C-N bonds cleavage. The energy consumption (EC) for FLO degradation ranged from 1.91 to 29.53 Wh/L, and the optimal practical conditions were obtained by the analysis of the calculated ratios of kFLO and EC. Moreover, La-Ti4O7 electrode maintained excellent removal efficiency of FLO (>93.5%) within 20 degradation cycles. This study suggested that La-Ti4O7 is a promising anodic material for the efficient treatment of FLO-polluted water.
中文翻译:

高活性 La 掺杂 Ti4O7 阳极电氧化氟苯尼考的研究
在这项研究中,通过简单的一步放电等离子烧结方法制备了一种新型活性La掺杂Ti 4 O 7 (La-Ti 4 O 7 )电极。表征结果表明,La 成功地结合到 Ti 4 O 7的晶格中,导致表面氧空位含量增加(从 26% 到 31%),析氧电位(从 2.24 到 2.75 V vs SCE),与原始 Ti 4 O 7相比,羟基自由基产率 [•OH,从 0.123 到 0.205 μmol/(min·cm 2 )] 和界面电荷转移速率。La-Ti 4 O 7电极实现了氟苯尼考(FLO,最广泛使用的抗生素之一)的有效阳极氧化,这主要是由于电产生的•OH介导的间接氧化。La-Ti 4 O 7电极对FLO的降解符合准一级动力学模型,1.60% La-Ti 4 O 7达到最佳降解速率常数( k FLO,0.021 min -1 )电极。此外,FLO 的降解效率随着电流密度的增加、pH 的降低和共存的 Cl -而增加,而与共存的 NO 3 -观察到相反的模式。. 通过 UPLC-MS/MS 鉴定出七种 FLO 降解产物。主要降解途径包括水解、羟基化、脱氯和CN键断裂。FLO降解的能耗(EC)范围为1.91~29.53 Wh/L,通过计算k FLO与EC的比值分析得出最佳实际条件。此外,La-Ti 4 O 7电极在 20 个降解循环内保持了优异的 FLO 去除效率(>93.5%)。该研究表明,La-Ti 4 O 7是一种很有前途的阳极材料,可用于有效处理 FLO 污染水。































 京公网安备 11010802027423号
京公网安备 11010802027423号