当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Access to 5-fluoroalkylated trisubstituted oxazoles via copper-catalyzed cyclization of α-fluoroalkyl-α-diazoketones with amides
Chemical Communications ( IF 4.3 ) Pub Date : 2022-03-22 , DOI: 10.1039/d2cc01057g Di Jiang 1 , Jingpei Jia 1 , Baiquan Wang 1, 2 , Bin Li 1
Chemical Communications ( IF 4.3 ) Pub Date : 2022-03-22 , DOI: 10.1039/d2cc01057g Di Jiang 1 , Jingpei Jia 1 , Baiquan Wang 1, 2 , Bin Li 1
Affiliation
|
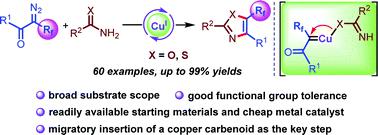
A novel copper-catalyzed cyclization of α-fluoroalkyl-α-diazoketones with (thio)amides has been developed. This mechanistically distinct protocol provides a robust and straightforward approach to construct 5-fluoroalkylated trisubstituted oxazoles and thiazoles with high efficiency and excellent functional group compatibility. Experimental studies suggest a mechanism involving imidate ligand migratory insertion of a copper carbenoid as the key step.
中文翻译:

通过铜催化 α-氟烷基-α-重氮酮与酰胺的环化获得 5-氟烷基化三取代恶唑
已经开发了一种新型铜催化的 α-氟烷基-α-重氮酮与(硫代)酰胺的环化反应。这种机制上不同的协议提供了一种强大而直接的方法来构建具有高效率和优异官能团兼容性的 5-氟烷基化三取代恶唑和噻唑。实验研究表明,作为关键步骤的亚胺配体迁移插入铜类碳烯的机制。
更新日期:2022-03-22
中文翻译:

通过铜催化 α-氟烷基-α-重氮酮与酰胺的环化获得 5-氟烷基化三取代恶唑
已经开发了一种新型铜催化的 α-氟烷基-α-重氮酮与(硫代)酰胺的环化反应。这种机制上不同的协议提供了一种强大而直接的方法来构建具有高效率和优异官能团兼容性的 5-氟烷基化三取代恶唑和噻唑。实验研究表明,作为关键步骤的亚胺配体迁移插入铜类碳烯的机制。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号