当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The alkoxylation effects on the excited-state intramolecular proton transfer behaviors for 2,6-bis(benzothiazolyl-2-yl)phenol fluorophore: A theoretical research
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2022-03-21 , DOI: 10.1002/poc.4341
Lijuan Shi 1 , Dapeng Yang 2
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2022-03-21 , DOI: 10.1002/poc.4341
Lijuan Shi 1 , Dapeng Yang 2
Affiliation
|
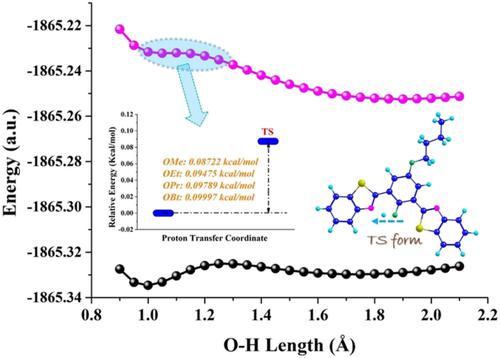
Given the unique luminescent properties, the excited-state intramolecular proton transfer (ESIPT) materials have attracted lots of eyes in recent years. In view of the promising highly efficient red-emitting material, the 2,6-bis(benzothiazolyl-2-yl)phenol (DBTP) under alkoxy groups at the 4-position reveals important properties. In this work, modification of DBTP fluorophore, that is, DBTP-OMe (methoxy), DBTP-OEt (ethoxy), DBTP-OPr (propoxy), and DBTP-OBt (butoxy) derivatives, have been explored theoretically. Based on the analyses of chemical structural variations and infrared (IR) vibrational spectra in both S0 and S1 states, the enhanced intramolecular hydrogen bonding interactions could be clearly found, which will promote the ESIPT tendency. Particularly, the DBTP-OMe with stronger hydrogen bond reveals the easier PT tendency upon photo-induced excitation. Paying attention to the changes of highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO), it could be found the charge transfer happens in the excited-state molecules, and the reorganization of charge densities should be conducive to promoting the ESIPT process. Simulated potential energy curves indicated the ultrafast ESIPT reaction occurs for DBTP-OMe, DBTP-OEt, DBTP-OPr, and DBTP-OBt derivatives. The searched transition state (TS) structures in S1-state reaction path suggested the derivatives with few alkyl groups might be more probe to occur the ESIPT reaction than the more alkyl ones for DBTP system.
中文翻译:

烷氧基化对 2,6-双(苯并噻唑基-2-基)苯酚荧光团激发态分子内质子转移行为的影响:一项理论研究
鉴于独特的发光特性,激发态分子内质子转移(ESIPT)材料近年来吸引了很多人的目光。鉴于有前景的高效发红光材料,4位烷氧基下的2,6-双(苯并噻唑基-2-基)苯酚(DBTP)显示出重要的性质。在这项工作中,从理论上探讨了 DBTP 荧光团的修饰,即 DBTP-OMe(甲氧基)、DBTP-OEt(乙氧基)、DBTP-OPr(丙氧基)和 DBTP-OBt(丁氧基)衍生物。基于对 S 0和 S 1中的化学结构变化和红外 (IR) 振动光谱的分析状态,可以清楚地发现增强的分子内氢键相互作用,这将促进ESIPT趋势。特别是,具有更强氢键的 DBTP-OMe 在光诱导激发下显示出更容易的 PT 趋势。关注最高占据分子轨道(HOMO)和最低未占据分子轨道(LUMO)的变化,可以发现电荷转移发生在激发态分子中,电荷密度的重组应该有利于促进ESIPT过程。模拟的势能曲线表明,DBTP-OMe、DBTP-OEt、DBTP-OPr 和 DBTP-OBt 衍生物发生超快 ESIPT 反应。S 1中搜索到的过渡态 (TS) 结构态反应路径表明,对于 DBTP 体系,具有较少烷基的衍生物可能比具有更多烷基的衍生物更容易发生 ESIPT 反应。
更新日期:2022-03-21
中文翻译:

烷氧基化对 2,6-双(苯并噻唑基-2-基)苯酚荧光团激发态分子内质子转移行为的影响:一项理论研究
鉴于独特的发光特性,激发态分子内质子转移(ESIPT)材料近年来吸引了很多人的目光。鉴于有前景的高效发红光材料,4位烷氧基下的2,6-双(苯并噻唑基-2-基)苯酚(DBTP)显示出重要的性质。在这项工作中,从理论上探讨了 DBTP 荧光团的修饰,即 DBTP-OMe(甲氧基)、DBTP-OEt(乙氧基)、DBTP-OPr(丙氧基)和 DBTP-OBt(丁氧基)衍生物。基于对 S 0和 S 1中的化学结构变化和红外 (IR) 振动光谱的分析状态,可以清楚地发现增强的分子内氢键相互作用,这将促进ESIPT趋势。特别是,具有更强氢键的 DBTP-OMe 在光诱导激发下显示出更容易的 PT 趋势。关注最高占据分子轨道(HOMO)和最低未占据分子轨道(LUMO)的变化,可以发现电荷转移发生在激发态分子中,电荷密度的重组应该有利于促进ESIPT过程。模拟的势能曲线表明,DBTP-OMe、DBTP-OEt、DBTP-OPr 和 DBTP-OBt 衍生物发生超快 ESIPT 反应。S 1中搜索到的过渡态 (TS) 结构态反应路径表明,对于 DBTP 体系,具有较少烷基的衍生物可能比具有更多烷基的衍生物更容易发生 ESIPT 反应。

































 京公网安备 11010802027423号
京公网安备 11010802027423号