当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights from computational analysis: how does the SARS-CoV-2 Delta (B.1.617.2) variant hijack ACE2 more effectively?
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2022-03-21 , DOI: 10.1039/d2cp00843b Danyang Xiong 1 , Xiaoyu Zhao 1 , Song Luo 1 , Lili Duan 1
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2022-03-21 , DOI: 10.1039/d2cp00843b Danyang Xiong 1 , Xiaoyu Zhao 1 , Song Luo 1 , Lili Duan 1
Affiliation
|
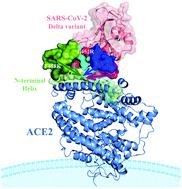
The SARS-CoV-2 Delta (B.1.617.2) variant was identified in India in October 2020, and it has quickly become the mainstream strain with strong toxicity and spread, posing great challenges to epidemic control. However, the molecular mechanism of its powerful infectivity remains unclear. It is meaningful to investigate the process of Delta variant's receptor-binding domain (RBD) binding to angiotensin-converting enzyme 2 (ACE2). Here, we performed three repeated molecular dynamics simulations for each system to avoid accidents, and the alanine scanning combined with the interaction entropy (ASIE) method was utilized to evaluate the binding free energy. Through the detailed energy and conformational analysis, the binding mechanism of the Delta variant was illustrated. The results showed that the existence of L452R and T478K mutations can trigger the effective hijacking of ACE2 by the Delta variant through the following three ways: (i) these two mutations can significantly enhance the electrostatic energy of the system by the introduction of two positively charged amino acids (Arg and Lys), thereby increasing the binding affinity of RBD and ACE2, (ii) the Loops 1, 3, and 4 in the receptor-binding motif (RBM) of RBD form a tighter conformation under the dominance of the T478K mutation, allowing ACE2 to be captured more effectively than the wild-type system, and (iii) these conformational changes lead to a more stable hydrogen bond in the Delta variant, which further ensures the stability of the binding. In addition, to explore the effect of mutations on the antibody, the key residues contributing to the changes in the binding ability of RBD in the Delta variant with the existing 42 neutralizing monoclonal antibodies (mAbs) have been preliminarily evaluated. The present study reveals the molecular mechanism for the increased infectivity of SARS-CoV-2 caused by mutations, and the key sites that cause antigenic changes were screened. It provides important theoretical insights for the development of novel targeted RBD drugs and antibodies.
中文翻译:

计算分析的见解:SARS-CoV-2 Delta (B.1.617.2) 变体如何更有效地劫持 ACE2?
SARS-CoV-2 Delta(B.1.617.2)变种于2020年10月在印度被发现,并迅速成为主流毒株,具有较强的毒性和传播性,对疫情防控构成巨大挑战。然而,其强大感染力的分子机制仍不清楚。研究Delta变体的受体结合域(RBD)与血管紧张素转换酶2(ACE2)结合的过程是有意义的。在这里,我们对每个系统进行了三次重复的分子动力学模拟以避免事故,并利用丙氨酸扫描结合相互作用熵(ASIE)方法来评估结合自由能。通过详细的能量和构象分析,阐明了Delta变体的结合机制。结果表明,L452R和T478K突变的存在可以通过以下三种方式触发Delta变体对ACE2的有效劫持:(i)这两种突变可以通过引入两个带正电荷的物质来显着增强系统的静电能。氨基酸(Arg 和 Lys),从而增加 RBD 和 ACE2 的结合亲和力,(ii) RBD 受体结合基序 (RBM) 中的环 1、3 和 4 在 T478K 的优势下形成更紧密的构象突变,允许 ACE2 比野生型系统更有效地被捕获,并且(iii)这些构象变化导致 Delta 变体中的氢键更稳定,这进一步确保了结合的稳定性。此外,为了探索突变对抗体的影响,已经初步评估了导致 Delta 变体中 RBD 与现有 42 种中和单克隆抗体 (mAb) 结合能力变化的关键残基。本研究揭示了突变导致SARS-CoV-2感染性增加的分子机制,并筛选了引起抗原变化的关键位点。它为新型靶向 RBD 药物和抗体的开发提供了重要的理论见解。
更新日期:2022-03-21
中文翻译:

计算分析的见解:SARS-CoV-2 Delta (B.1.617.2) 变体如何更有效地劫持 ACE2?
SARS-CoV-2 Delta(B.1.617.2)变种于2020年10月在印度被发现,并迅速成为主流毒株,具有较强的毒性和传播性,对疫情防控构成巨大挑战。然而,其强大感染力的分子机制仍不清楚。研究Delta变体的受体结合域(RBD)与血管紧张素转换酶2(ACE2)结合的过程是有意义的。在这里,我们对每个系统进行了三次重复的分子动力学模拟以避免事故,并利用丙氨酸扫描结合相互作用熵(ASIE)方法来评估结合自由能。通过详细的能量和构象分析,阐明了Delta变体的结合机制。结果表明,L452R和T478K突变的存在可以通过以下三种方式触发Delta变体对ACE2的有效劫持:(i)这两种突变可以通过引入两个带正电荷的物质来显着增强系统的静电能。氨基酸(Arg 和 Lys),从而增加 RBD 和 ACE2 的结合亲和力,(ii) RBD 受体结合基序 (RBM) 中的环 1、3 和 4 在 T478K 的优势下形成更紧密的构象突变,允许 ACE2 比野生型系统更有效地被捕获,并且(iii)这些构象变化导致 Delta 变体中的氢键更稳定,这进一步确保了结合的稳定性。此外,为了探索突变对抗体的影响,已经初步评估了导致 Delta 变体中 RBD 与现有 42 种中和单克隆抗体 (mAb) 结合能力变化的关键残基。本研究揭示了突变导致SARS-CoV-2感染性增加的分子机制,并筛选了引起抗原变化的关键位点。它为新型靶向 RBD 药物和抗体的开发提供了重要的理论见解。













































 京公网安备 11010802027423号
京公网安备 11010802027423号