European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2022-03-21 , DOI: 10.1016/j.ejmech.2022.114281 Yi Zhao 1 , Yao Peng 2 , Zhongzhen Yang 2 , Jiaqi Lu 2 , Ru Li 2 , Yuesen Shi 2 , Yaxin Du 2 , Ze Zhao 3 , Li Hai 2 , Yong Wu 2
|
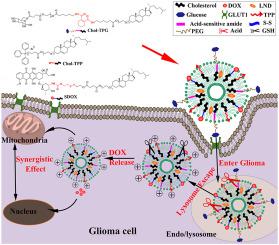
To synergistically treat glioma with a combination chemotherapy, we design and prepare novel cascade-targeted liposomes (Lip-TPGS) using glucose and triphenylphosphonium (TPP) as targeting moieties, which could intelligently deliver redox-sensitive doxorubicin (DOX) prodrugs (SDOX) and chemotherapeutic sensitizer lonidamine (LND). The pH-responsive ligand Chol-TPG modified by PEGylated glucose can overcome the blood-brain barrier and reach tumor cells. Combined with the modification of mitochondria targeting ligand (Chol-TPP), Lip-TPGS are endowed with pH-responsive charge regulation function and multi-stage targeting abilities. After triggered by the excessive glutathione in tumor cells, Lip-TPGS could sufficiently release the parent drugs DOX, which would significantly reduce side effects without compromising anti-glioma efficacy. Therefore, Lip-TPGS possess these characteristics: good pharmacokinetic behavior, superior brain targeting ability, specific tumor recognition and internalization capability, and strong endo/lysosome escaping and mitochondria targeting potential. Furthermore, Lip-TPGS exhibit significant advantages on anti-glioma by inhibiting proliferation, promoting apoptosis, inducing mitochondria dysfunction, inhibiting migration and invasion, prolonging the survival time, narrowing tumor areas, limiting lung metastasis, and reducing toxicity to normal organs. In summary, Lip-TPGS, with cascade targeting abilities from tissue/cell to organelle levels and highly controlled drug release properties, would become a promising drug delivery system for glioma treatment.
中文翻译:

pH-氧化还原响应级联靶向脂质体智能递送多柔比星前药和洛尼达明治疗胶质瘤
为了与联合化疗协同治疗胶质瘤,我们设计并制备了新型级联靶向脂质体 (Lip-TPGS),使用葡萄糖和三苯基鏻 (TPP) 作为靶向部分,可以智能地递送氧化还原敏感的多柔比星 (DOX) 前药 (SDOX) 和化疗增敏剂洛尼达明(LND)。PEG化葡萄糖修饰的pH响应性配体Chol-TPG可以克服血脑屏障到达肿瘤细胞。结合线粒体靶向配体(Chol-TPP)的修饰,Lip-TPGS具有pH响应性电荷调节功能和多阶段靶向能力。在肿瘤细胞中过量的谷胱甘肽触发后,Lip-TPGS可以充分释放母体药物DOX,在不影响抗胶质瘤疗效的情况下显着降低副作用。所以,Lip-TPGS具有以下特点:良好的药代动力学行为、优越的脑靶向能力、特异性的肿瘤识别和内化能力以及强大的内/溶酶体逃逸和线粒体靶向潜力。此外,Lip-TPGS 通过抑制增殖、促进细胞凋亡、诱导线粒体功能障碍、抑制迁移和侵袭、延长存活时间、缩小肿瘤区域、限制肺转移和降低对正常器官的毒性等方面在抗胶质瘤方面表现出显着优势。总之,Lip-TPGS 具有从组织/细胞到细胞器水平的级联靶向能力和高度可控的药物释放特性,将成为一种有前途的神经胶质瘤治疗药物输送系统。特异性肿瘤识别和内化能力,以及强大的内/溶酶体逃逸和线粒体靶向潜力。此外,Lip-TPGS 通过抑制增殖、促进细胞凋亡、诱导线粒体功能障碍、抑制迁移和侵袭、延长存活时间、缩小肿瘤区域、限制肺转移和降低对正常器官的毒性等方面在抗胶质瘤方面表现出显着优势。总之,Lip-TPGS 具有从组织/细胞到细胞器水平的级联靶向能力和高度可控的药物释放特性,将成为一种有前途的神经胶质瘤治疗药物输送系统。特异性肿瘤识别和内化能力,以及强大的内/溶酶体逃逸和线粒体靶向潜力。此外,Lip-TPGS 通过抑制增殖、促进细胞凋亡、诱导线粒体功能障碍、抑制迁移和侵袭、延长存活时间、缩小肿瘤区域、限制肺转移和降低对正常器官的毒性等方面在抗胶质瘤方面表现出显着优势。总之,Lip-TPGS 具有从组织/细胞到细胞器水平的级联靶向能力和高度可控的药物释放特性,将成为一种有前途的神经胶质瘤治疗药物输送系统。Lip-TPGS通过抑制增殖、促进细胞凋亡、诱导线粒体功能障碍、抑制迁移和侵袭、延长存活时间、缩小肿瘤区域、限制肺转移和降低对正常器官的毒性等方面在抗胶质瘤方面表现出显着优势。总之,Lip-TPGS 具有从组织/细胞到细胞器水平的级联靶向能力和高度可控的药物释放特性,将成为一种有前途的神经胶质瘤治疗药物输送系统。Lip-TPGS通过抑制增殖、促进细胞凋亡、诱导线粒体功能障碍、抑制迁移和侵袭、延长存活时间、缩小肿瘤区域、限制肺转移和降低对正常器官的毒性等方面在抗胶质瘤方面表现出显着优势。总之,Lip-TPGS 具有从组织/细胞到细胞器水平的级联靶向能力和高度可控的药物释放特性,将成为一种有前途的神经胶质瘤治疗药物输送系统。































 京公网安备 11010802027423号
京公网安备 11010802027423号