当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
How Photoactivation Triggers Protochlorophyllide Reduction: Computational Evidence of a Stepwise Hydride Transfer during Chlorophyll Biosynthesis
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-03-21 , DOI: 10.1021/acscatal.2c00866 Linus O Johannissen 1 , Aoife Taylor 1 , Samantha J O Hardman 1 , Derren J Heyes 1 , Nigel S Scrutton 1 , Sam Hay 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-03-21 , DOI: 10.1021/acscatal.2c00866 Linus O Johannissen 1 , Aoife Taylor 1 , Samantha J O Hardman 1 , Derren J Heyes 1 , Nigel S Scrutton 1 , Sam Hay 1
Affiliation
|
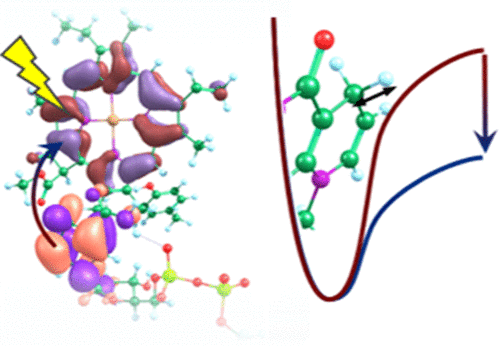
The photochemical reaction catalyzed by enzyme protochlorophyllide oxidoreductase (POR), a rare example of a photoactivated enzyme, is a crucial step during chlorophyll biosynthesis and involves the fastest known biological hydride transfer. Structures of the enzyme with bound substrate protochlorophyllide (PChlide) and coenzyme nicotinamide adenine dinucleotide phosphate (NADPH) have recently been published, opening up the possibility of using computational approaches to provide a comprehensive understanding of the excited state chemistry. Herein, we propose a complete mechanism for the photochemistry between PChlide and NADPH based on density functional theory (DFT) and time-dependent DFT calculations that is consistent with recent experimental data. In this multi-step mechanism, photoexcitation of PChlide leads to electron transfer from NADPH to PChlide, which in turn facilitates hydrogen atom transfer by weakening the breaking C–H bond. This work rationalizes how photoexcitation facilitates hydride transfer in POR and has more general implications for biological hydride transfer reactions.
中文翻译:

光活化如何触发原叶绿素内酯还原:叶绿素生物合成过程中逐步氢化物转移的计算证据
原叶绿素氧化还原酶 (POR) 是一种罕见的光活化酶,它催化的光化学反应是叶绿素生物合成过程中的关键步骤,涉及已知最快的生物氢化物转移。具有结合底物原叶绿素内酯 (PChlide) 和辅酶烟酰胺腺嘌呤二核苷酸磷酸 (NADPH) 的酶的结构最近已发表,开启了使用计算方法全面了解激发态化学的可能性。在此,我们基于密度泛函理论(DFT)和与最新实验数据一致的时间依赖性DFT计算,提出了PChlide和NADPH之间光化学的完整机制。在这个多步骤机制中,PChlide的光激发导致电子从NADPH转移到PChlide,这反过来又通过削弱断裂的C-H键来促进氢原子转移。这项工作合理化了光激发如何促进 POR 中的氢化物转移,并对生物氢化物转移反应具有更普遍的影响。
更新日期:2022-03-21
中文翻译:

光活化如何触发原叶绿素内酯还原:叶绿素生物合成过程中逐步氢化物转移的计算证据
原叶绿素氧化还原酶 (POR) 是一种罕见的光活化酶,它催化的光化学反应是叶绿素生物合成过程中的关键步骤,涉及已知最快的生物氢化物转移。具有结合底物原叶绿素内酯 (PChlide) 和辅酶烟酰胺腺嘌呤二核苷酸磷酸 (NADPH) 的酶的结构最近已发表,开启了使用计算方法全面了解激发态化学的可能性。在此,我们基于密度泛函理论(DFT)和与最新实验数据一致的时间依赖性DFT计算,提出了PChlide和NADPH之间光化学的完整机制。在这个多步骤机制中,PChlide的光激发导致电子从NADPH转移到PChlide,这反过来又通过削弱断裂的C-H键来促进氢原子转移。这项工作合理化了光激发如何促进 POR 中的氢化物转移,并对生物氢化物转移反应具有更普遍的影响。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号