Science China Materials ( IF 6.8 ) Pub Date : 2021-11-22 , DOI: 10.1007/s40843-021-1827-x Yining Jia 1 , Yaokun Ye 1 , Jiahua Liu 1 , Shisheng Zheng 1 , Weicheng Lin 1 , Zhu Wang 1 , Shunning Li 1 , Feng Pan 1 , Jiaxin Zheng 1, 2
|
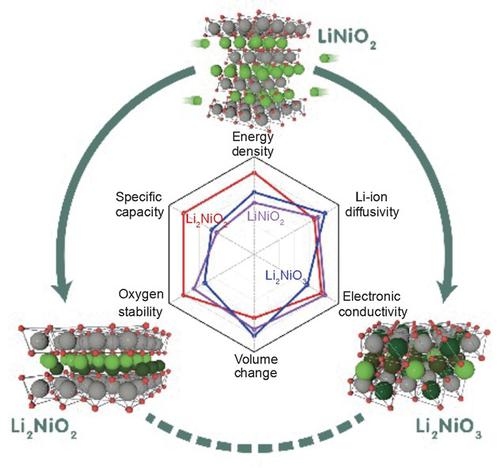
The development of next-generation layered oxide cathodes for high-energy-density electrical vehicle Li-ion batteries (LIBs) is an urgent topic. The existing method is achieved by continuously increasing the Ni contents of Ni-based layered oxides, but it has been limited to LiNiO2. To break this limit and attain increased energy densities, a promising strategy, which involves the introduction of excess Li ions into transition metal (TM) layers to form Li-excess compounds Li2MO3 (M is a TM cation), has attracted enormous interest recently. However, another strategy, which has been neglected in recent years, involves the insertion of an extra layer of Li ions between the TM and original Li layers to form Li2MO2. In this study, typical reversible Li2NiO3 and 1T-Li2NiO2 were selected as two representative cathodes to break the limit of LiNiO2, thereby availing comprehensive comparison with LiNiO2 regarding their overall properties as cathodes from a theoretical perspective. Interestingly, dissimilar to the Ni3+/Ni4+ monoelectron cationic redox associated with LiNiO2, a polaronic anionic redox reaction occurs in Li2NiO3, while a reversible Ni2+/Ni4+ double-electron redox reaction accompanied by insulator-metal transition occurs in Li2NiO2. Owing to this double-electron cationic activity, Li2NiO2 exhibits absolute advantages over the other two materials (LiNiO2 and Li2NiO3) as cathodes for LIBs in terms of the capacity, energy density, electronic conductivity, and thermal stability, thus rendering it the most promising candidate for next-generation layered oxide cathodes with high energy densities to break the limit of LiNiO2.
中文翻译:

打破LiNiO2的能量密度极限:Li2NiO3还是Li2NiO2?
开发用于高能量密度电动汽车锂离子电池(LIB)的下一代层状氧化物正极是一个紧迫的课题。现有方法是通过不断增加Ni基层状氧化物的Ni含量来实现的,但仅限于LiNiO 2。为了打破这一限制并获得更高的能量密度,一种有前途的策略,包括将过量的锂离子引入过渡金属 (TM) 层以形成锂过量化合物 Li 2 MO 3 (M 是一种 TM 阳离子),已经吸引了大量的最近感兴趣。然而,近年来被忽视的另一种策略涉及在 TM 和原始 Li 层之间插入额外的锂离子层以形成 Li 2 MO 2. 本研究选择了典型的可逆Li 2 NiO 3和1T-Li 2 NiO 2作为两种代表性正极,突破了LiNiO 2的限制,从而从理论角度对它们作为正极的整体性能进行了综合比较。有趣的是,与与 LiNiO 2相关的 Ni 3+ /Ni 4+单电子阳离子氧化还原不同,Li 2 NiO 3发生极化阴离子氧化还原反应,而可逆的 Ni 2+ /Ni 4+Li 2 NiO 2中发生了伴随绝缘体-金属转变的双电子氧化还原反应。由于这种双电子阳离子活性,Li 2 NiO 2在容量、能量密度、电子电导率和热稳定性方面作为锂离子电池正极材料比其他两种材料(LiNiO 2和 Li 2 NiO 3 )具有绝对优势,因此使其成为下一代高能量密度层状氧化物正极最有希望突破LiNiO 2极限的候选材料。

































 京公网安备 11010802027423号
京公网安备 11010802027423号