当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Inclusion complexes of the macrocycle nonactin with benchmark protonated amines: aniline and serine
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2022-03-18 , DOI: 10.1039/d2cp00264g Juan Ramón Avilés-Moreno 1 , Francisco Gámez 2 , Giel Berden 3 , Jos Oomens 3 , Bruno Martínez-Haya 4
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2022-03-18 , DOI: 10.1039/d2cp00264g Juan Ramón Avilés-Moreno 1 , Francisco Gámez 2 , Giel Berden 3 , Jos Oomens 3 , Bruno Martínez-Haya 4
Affiliation
|
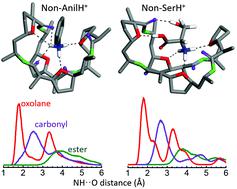
The biological activity of the macrocycle nonactin is intimately related to its ionophore properties and ability to act as a selective cation carrier. While the focus of most investigations on nonactin has been on the binding of metal cations and small molecular ions, this study pursues the characterization of its inclusion complexes with primary amines with bulky structured side groups of different polarity. To this end, the complexes of nonactin with aniline and with the amino acid L-serine, both in protonated form, are considered as case studies and their relevant coordination arrangements are assessed by means of infrared action spectroscopy, quantum chemical density functional theory and Born–Oppenheimer molecular dynamics. The study suggests that the oxygen atoms from the oxolane (tetrahydrofuran) groups of nonactin constitute the preferential docking sites of the ammonium moiety of the guest cation, although conformational constraints promote interactions with the ester carbonyl backbone groups. In the aniline complex, the benzyl side ring is oriented outwards from the cavity, whereas in the case of L-serine, the side carboxylic acid and alcohol groups participate actively in the coordination process. Interestingly, the accommodation of L-serine is favoured when nonactin adopts an enantiomeric-selective folding, that promotes the tripodal coordination of the protonated amine group with oxolane rings from three nonactinic acid blocks with enantiomeric sequence (+)-(−)-(+), which allows for a facile coordination of the serine side groups. This is recognized as a general feature associated with the alternation of chiral domains in globally achiral natural nonactin, yielding mirror-symmetric complexes with the enantiomers of chiral amines.
中文翻译:

大环非肌动蛋白与基准质子化胺的包合物:苯胺和丝氨酸
大环非肌动蛋白的生物活性与其离子载体性质和作为选择性阳离子载体的能力密切相关。虽然对非肌动蛋白的大多数研究的重点是金属阳离子和小分子离子的结合,但本研究致力于表征其与具有不同极性的庞大结构侧基的伯胺的包合物。为此,nonactin 与苯胺和氨基酸L的复合物质子化形式的-丝氨酸被视为案例研究,并通过红外作用光谱、量子化学密度泛函理论和 Born-Oppenheimer 分子动力学评估其相关配位排列。该研究表明,非肌动蛋白的氧杂环戊烷(四氢呋喃)基团中的氧原子构成了客体阳离子铵部分的优先对接位点,尽管构象限制促进了与酯羰基骨架基团的相互作用。在苯胺配合物中,苄基侧环从空腔向外定向,而在L-丝氨酸的情况下,侧羧酸和醇基团积极参与配位过程。有趣的是,L的住宿当非肌动蛋白采用对映体选择性折叠时,丝氨酸受到青睐,这促进了质子化胺基与来自三个具有对映体序列 (+)-(-)-(+) 的非光化酸嵌段的氧杂环戊烷环的三足配位,这允许丝氨酸侧基的轻松协调。这被认为是与全局非手性天然非肌动蛋白中手性结构域交替相关的一般特征,产生与手性胺的对映异构体的镜像对称复合物。
更新日期:2022-03-18
中文翻译:

大环非肌动蛋白与基准质子化胺的包合物:苯胺和丝氨酸
大环非肌动蛋白的生物活性与其离子载体性质和作为选择性阳离子载体的能力密切相关。虽然对非肌动蛋白的大多数研究的重点是金属阳离子和小分子离子的结合,但本研究致力于表征其与具有不同极性的庞大结构侧基的伯胺的包合物。为此,nonactin 与苯胺和氨基酸L的复合物质子化形式的-丝氨酸被视为案例研究,并通过红外作用光谱、量子化学密度泛函理论和 Born-Oppenheimer 分子动力学评估其相关配位排列。该研究表明,非肌动蛋白的氧杂环戊烷(四氢呋喃)基团中的氧原子构成了客体阳离子铵部分的优先对接位点,尽管构象限制促进了与酯羰基骨架基团的相互作用。在苯胺配合物中,苄基侧环从空腔向外定向,而在L-丝氨酸的情况下,侧羧酸和醇基团积极参与配位过程。有趣的是,L的住宿当非肌动蛋白采用对映体选择性折叠时,丝氨酸受到青睐,这促进了质子化胺基与来自三个具有对映体序列 (+)-(-)-(+) 的非光化酸嵌段的氧杂环戊烷环的三足配位,这允许丝氨酸侧基的轻松协调。这被认为是与全局非手性天然非肌动蛋白中手性结构域交替相关的一般特征,产生与手性胺的对映异构体的镜像对称复合物。















































 京公网安备 11010802027423号
京公网安备 11010802027423号