当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The enantioselective total syntheses of (+)-7-oxohinokinin, (+)-7-oxoarcitin, (+)-conicaol B and (−)-isopolygamain
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2022-03-17 , DOI: 10.1039/d2ob00336h Emily K Paulin 1, 2 , Euphemia Leung 3 , Lisa I Pilkington 1 , David Barker 1, 2
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2022-03-17 , DOI: 10.1039/d2ob00336h Emily K Paulin 1, 2 , Euphemia Leung 3 , Lisa I Pilkington 1 , David Barker 1, 2
Affiliation
|
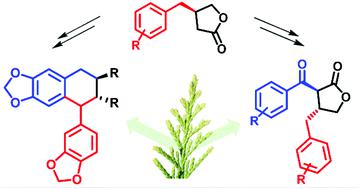
A flexible approach to C7 keto dibenzyl butyrolactone lignans was developed and the synthesis of several natural products and their related derivatives is described herein. The developed pathway proceeds through enantioenriched β-substituted butyrolactones, from which facile aldol addition and subsequent oxidation affords the desired benzylic ketone moiety. This methodology was used to complete the first enantioselective total syntheses of three natural products, (+)-7-oxohinokinin, (+)-7-oxoarcitin and (+)-conicaol B, and a further five analogues. The utility of this method was further demonstrated through a 1–2 step modification to access another class of natural product, aryltetralin lignans, allowing the asymmetric total synthesis of (−)-isopolygamain and a polygamain derivative. Anti-proliferative testing determined (−)-isopolygamain was the most active of the compounds prepared, with IC50 values of 2.95 ± 0.61 μM and 4.65 ± 0.68 μM against MDA-MB-231 (triple negative breast cancer) and HCT-116 (colon cancer) cell lines, respectively.
中文翻译:

(+)-7-oxoarcitin、(+)-7-oxoarcitin、(+)-conicaol B和(-)-isopolygamain的对映选择性全合成
开发了 C7 酮基二苄基丁内酯木脂素的灵活方法,本文描述了几种天然产物及其相关衍生物的合成。开发的途径通过对映体富集的 β-取代丁内酯进行,从中容易进行醛醇加成和随后的氧化,从而提供所需的苄基酮部分。该方法用于完成三种天然产物 (+)-7-oxoarcitin、(+)-7-oxoarcitin 和 (+)-conicaol B 以及另外五种类似物的首次对映选择性全合成。该方法的实用性通过 1-2 步修改得到进一步证明,以获取另一类天然产物芳基四氢化萘木脂素,允许 (-)-isopolygamain 和 polygamain 衍生物的不对称全合成。针对 MDA-MB-231(三阴性乳腺癌)和 HCT-116(结肠癌)细胞系的50 个值分别为 2.95 ± 0.61 μM 和 4.65 ± 0.68 μM。
更新日期:2022-03-17
中文翻译:

(+)-7-oxoarcitin、(+)-7-oxoarcitin、(+)-conicaol B和(-)-isopolygamain的对映选择性全合成
开发了 C7 酮基二苄基丁内酯木脂素的灵活方法,本文描述了几种天然产物及其相关衍生物的合成。开发的途径通过对映体富集的 β-取代丁内酯进行,从中容易进行醛醇加成和随后的氧化,从而提供所需的苄基酮部分。该方法用于完成三种天然产物 (+)-7-oxoarcitin、(+)-7-oxoarcitin 和 (+)-conicaol B 以及另外五种类似物的首次对映选择性全合成。该方法的实用性通过 1-2 步修改得到进一步证明,以获取另一类天然产物芳基四氢化萘木脂素,允许 (-)-isopolygamain 和 polygamain 衍生物的不对称全合成。针对 MDA-MB-231(三阴性乳腺癌)和 HCT-116(结肠癌)细胞系的50 个值分别为 2.95 ± 0.61 μM 和 4.65 ± 0.68 μM。






























 京公网安备 11010802027423号
京公网安备 11010802027423号