当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparative Studies to Uncover Mechanisms of Action of N-(1,3,4-Oxadiazol-2-yl)benzamide Containing Antibacterial Agents
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2022-03-17 , DOI: 10.1021/acsinfecdis.1c00613 George A Naclerio 1 , Kenneth I Onyedibe 1, 2 , Caroline W Karanja 1 , Uma K Aryal 3, 4 , Herman O Sintim 1, 2
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2022-03-17 , DOI: 10.1021/acsinfecdis.1c00613 George A Naclerio 1 , Kenneth I Onyedibe 1, 2 , Caroline W Karanja 1 , Uma K Aryal 3, 4 , Herman O Sintim 1, 2
Affiliation
|
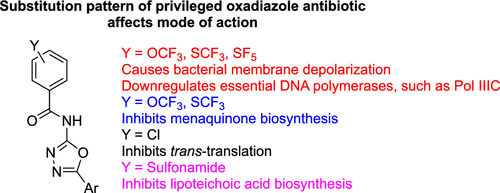
Drug-resistant bacterial pathogens still cause high levels of mortality annually despite the availability of many antibiotics. Methicillin-resistant Staphylococcus aureus (MRSA) is especially problematic, and the rise in resistance to front-line treatments like vancomycin and linezolid calls for new chemical modalities to treat chronic and relapsing MRSA infections. Halogenated N-(1,3,4-oxadiazol-2-yl)benzamides are an interesting class of antimicrobial agents, which have been described by multiple groups to be effective against different bacterial pathogens. The modes of action of a few N-(1,3,4-oxadiazol-2-yl)benzamides have been elucidated. For example, oxadiazoles KKL-35 and MBX-4132 have been described as inhibitors of trans-translation (a ribosome rescue pathway), while HSGN-94 was shown to inhibit lipoteichoic acid (LTA). However, other similarly halogenated N-(1,3,4-oxadiazol-2-yl)benzamides neither inhibit trans-translation nor LTA biosynthesis but are potent antimicrobial agents. For example, HSGN-220, -218, and -144 are N-(1,3,4-oxadiazol-2-yl)benzamides that are modified with OCF3, SCF3, or SF5 and have remarkable minimum inhibitory concentrations ranging from 1 to 0.06 μg/mL against MRSA clinical isolates and show a low propensity to develop resistance to MRSA over 30 days. The mechanism of action of these highly potent oxadiazoles is however unknown. To provide insights into how these halogenated N-(1,3,4-oxadiazol-2-yl)benzamides inhibit bacterial growth, we performed global proteomics and RNA expression analysis of some essential genes of S. aureus treated with HSGN-220, -218, and -144. These studies revealed that the oxadiazoles HSGN-220, -218, and -144 are multitargeting antibiotics that regulate menaquinone biosynthesis and other essential proteins like DnaX, Pol IIIC, BirA, LexA, and DnaC. In addition, these halogenated N-(1,3,4-oxadiazol-2-yl)benzamides were able to depolarize bacterial membranes and regulate siderophore biosynthesis and heme regulation. Iron starvation appears to be part of the mechanism of action that led to bacterial killing. This study demonstrates that N-(1,3,4-oxadiazol-2-yl)benzamides are indeed privileged scaffolds for the development of antibacterial agents and that subtle modifications lead to changes to the mechanism of action.
中文翻译:

比较研究揭示含 N-(1,3,4-Oxadiazol-2-yl)benzamide 抗菌剂的作用机制
尽管有许多抗生素可供使用,但耐药细菌病原体每年仍会导致高水平的死亡率。耐甲氧西林金黄色葡萄球菌(MRSA) 尤其成问题,对万古霉素和利奈唑胺等一线治疗药物的耐药性增加需要新的化学方法来治疗慢性和复发性 MRSA 感染。卤化N -(1,3,4-oxadiazol-2-yl)benzamides 是一类有趣的抗菌剂,多个研究小组已将其描述为对不同的细菌病原体有效。已经阐明了一些N -(1,3,4-oxadiazol-2-yl)benzamides 的作用模式。例如,恶二唑 KKL-35 和 MBX-4132 已被描述为反式抑制剂-翻译(核糖体救援途径),而 HSGN-94 显示可抑制脂磷壁酸 (LTA)。然而,其他类似的卤化N -(1,3,4-oxadiazol-2-yl)benzamides 既不抑制反式翻译也不抑制 LTA 生物合成,而是有效的抗菌剂。例如,HSGN-220、-218和-144是用 OCF 3、SCF 3或 SF 5改性的N -(1,3,4-oxadiazol-2-yl)benzamides并且对 MRSA 临床分离株具有 1 至 0.06 μg/mL 的显着最低抑制浓度,并且在 30 天内显示出对 MRSA 产生耐药性的低倾向。然而,这些高效恶二唑的作用机制尚不清楚。为了深入了解这些卤化N -(1,3,4-恶二唑-2-基)苯甲酰胺如何抑制细菌生长,我们对用HSGN-220处理的金黄色葡萄球菌的一些必需基因进行了全局蛋白质组学和 RNA 表达分析,- 218和-144。这些研究表明,恶二唑HSGN-220、-218和-144是调节甲基萘醌生物合成和其他必需蛋白质(如 DnaX、Pol IIIC、BirA、LexA 和 DnaC)的多靶点抗生素。此外,这些卤代N -(1,3,4-oxadiazol-2-yl)benzamides 能够使细菌膜去极化并调节铁载体生物合成和血红素调节。缺铁似乎是导致细菌死亡的作用机制的一部分。这项研究表明,N -(1,3,4-oxadiazol-2-yl)benzamides 确实是开发抗菌剂的特殊支架,细微的修饰会导致作用机制发生变化。
更新日期:2022-03-17
中文翻译:

比较研究揭示含 N-(1,3,4-Oxadiazol-2-yl)benzamide 抗菌剂的作用机制
尽管有许多抗生素可供使用,但耐药细菌病原体每年仍会导致高水平的死亡率。耐甲氧西林金黄色葡萄球菌(MRSA) 尤其成问题,对万古霉素和利奈唑胺等一线治疗药物的耐药性增加需要新的化学方法来治疗慢性和复发性 MRSA 感染。卤化N -(1,3,4-oxadiazol-2-yl)benzamides 是一类有趣的抗菌剂,多个研究小组已将其描述为对不同的细菌病原体有效。已经阐明了一些N -(1,3,4-oxadiazol-2-yl)benzamides 的作用模式。例如,恶二唑 KKL-35 和 MBX-4132 已被描述为反式抑制剂-翻译(核糖体救援途径),而 HSGN-94 显示可抑制脂磷壁酸 (LTA)。然而,其他类似的卤化N -(1,3,4-oxadiazol-2-yl)benzamides 既不抑制反式翻译也不抑制 LTA 生物合成,而是有效的抗菌剂。例如,HSGN-220、-218和-144是用 OCF 3、SCF 3或 SF 5改性的N -(1,3,4-oxadiazol-2-yl)benzamides并且对 MRSA 临床分离株具有 1 至 0.06 μg/mL 的显着最低抑制浓度,并且在 30 天内显示出对 MRSA 产生耐药性的低倾向。然而,这些高效恶二唑的作用机制尚不清楚。为了深入了解这些卤化N -(1,3,4-恶二唑-2-基)苯甲酰胺如何抑制细菌生长,我们对用HSGN-220处理的金黄色葡萄球菌的一些必需基因进行了全局蛋白质组学和 RNA 表达分析,- 218和-144。这些研究表明,恶二唑HSGN-220、-218和-144是调节甲基萘醌生物合成和其他必需蛋白质(如 DnaX、Pol IIIC、BirA、LexA 和 DnaC)的多靶点抗生素。此外,这些卤代N -(1,3,4-oxadiazol-2-yl)benzamides 能够使细菌膜去极化并调节铁载体生物合成和血红素调节。缺铁似乎是导致细菌死亡的作用机制的一部分。这项研究表明,N -(1,3,4-oxadiazol-2-yl)benzamides 确实是开发抗菌剂的特殊支架,细微的修饰会导致作用机制发生变化。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号