当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The First-in-Human Synthetic Glycan-Based Conjugate Vaccine Candidate against Shigella
ACS Central Science ( IF 12.7 ) Pub Date : 2022-03-17 , DOI: 10.1021/acscentsci.1c01479 Robert M F van der Put 1 , Carolien Smitsman 1 , Alex de Haan 1 , Martin Hamzink 1 , Hans Timmermans 1 , Joost Uittenbogaard 1 , Janny Westdijk 1 , Michiel Stork 1 , Olga Ophorst 1 , Françoise Thouron 2 , Catherine Guerreiro 3 , Philippe J Sansonetti 2, 4 , Armelle Phalipon 2 , Laurence A Mulard 3
ACS Central Science ( IF 12.7 ) Pub Date : 2022-03-17 , DOI: 10.1021/acscentsci.1c01479 Robert M F van der Put 1 , Carolien Smitsman 1 , Alex de Haan 1 , Martin Hamzink 1 , Hans Timmermans 1 , Joost Uittenbogaard 1 , Janny Westdijk 1 , Michiel Stork 1 , Olga Ophorst 1 , Françoise Thouron 2 , Catherine Guerreiro 3 , Philippe J Sansonetti 2, 4 , Armelle Phalipon 2 , Laurence A Mulard 3
Affiliation
|
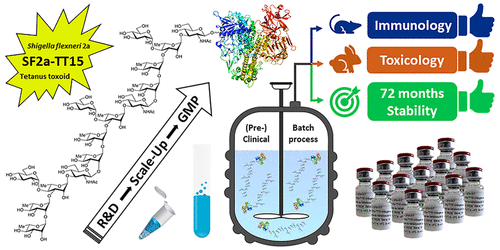
Shigella, the causative agent of shigellosis, is among the main causes of diarrheal diseases with still a high morbidity in low-income countries. Relying on chemical synthesis, we implemented a multidisciplinary strategy to design SF2a-TT15, an original glycoconjugate vaccine candidate targeting Shigella flexneri 2a (SF2a). Whereas the SF2a O-antigen features nonstoichiometric O-acetylation, SF2a-TT15 is made of a synthetic 15mer oligosaccharide, corresponding to three non-O-acetylated repeats, linked at its reducing end to tetanus toxoid by means of a thiol-maleimide spacer. We report on the scale-up feasibility under GMP conditions of a high yielding bioconjugation process established to ensure a reproducible and controllable glycan/protein ratio. Preclinical and clinical batches complying with specifications from ICH guidelines, WHO recommendations for polysaccharide conjugate vaccines, and (non)compendial tests were produced. The obtained SF2a-TT15 vaccine candidate passed all toxicity-related criteria, was immunogenic in rabbits, and elicited bactericidal antibodies in mice. Remarkably, the induced IgG antibodies recognized a large panel of SF2a circulating strains. These preclinical data have paved the way forward to the first-in-human study for SF2a-TT15, demonstrating safety and immunogenicity. This contribution discloses the yet unreported feasibility of the GMP synthesis of conjugate vaccines featuring a unique homogeneous synthetic glycan hapten fine-tuned to protect against an infectious disease.
中文翻译:

首个针对志贺氏菌的人体合成多糖共轭疫苗候选者
志贺氏菌是志贺氏菌病的病原体,是腹泻病的主要原因之一,在低收入国家的发病率仍然很高。依托化学合成,我们实施了多学科策略设计SF2a-TT15,这是一种针对福氏志贺氏菌的原始糖结合疫苗候选物2a (SF2a)。SF2a O-抗原具有非化学计量的 O-乙酰化,而 SF2a-TT15 由合成的 15 聚体寡糖组成,对应于三个非 O-乙酰化重复序列,在其还原端通过硫醇-马来酰亚胺间隔物连接到破伤风类毒素。我们报告了在 GMP 条件下为确保可重现和可控的聚糖/蛋白质比率而建立的高产生物偶联工艺的放大可行性。生产了符合 ICH 指南规范、WHO 多糖结合疫苗建议和(非)药典试验规范的临床前和临床批次。获得的 SF2a-TT15 候选疫苗通过了所有毒性相关标准,在兔子中具有免疫原性,并在小鼠中引发杀菌抗体。值得注意的是,诱导的 IgG 抗体可识别大量 SF2a 循环菌株。这些临床前数据为 SF2a-TT15 的首次人体研究铺平了道路,证明了安全性和免疫原性。这一贡献揭示了结合疫苗的 GMP 合成尚未报道的可行性,该疫苗具有独特的均质合成聚糖半抗原,经过微调以预防传染病。
更新日期:2022-03-17
中文翻译:

首个针对志贺氏菌的人体合成多糖共轭疫苗候选者
志贺氏菌是志贺氏菌病的病原体,是腹泻病的主要原因之一,在低收入国家的发病率仍然很高。依托化学合成,我们实施了多学科策略设计SF2a-TT15,这是一种针对福氏志贺氏菌的原始糖结合疫苗候选物2a (SF2a)。SF2a O-抗原具有非化学计量的 O-乙酰化,而 SF2a-TT15 由合成的 15 聚体寡糖组成,对应于三个非 O-乙酰化重复序列,在其还原端通过硫醇-马来酰亚胺间隔物连接到破伤风类毒素。我们报告了在 GMP 条件下为确保可重现和可控的聚糖/蛋白质比率而建立的高产生物偶联工艺的放大可行性。生产了符合 ICH 指南规范、WHO 多糖结合疫苗建议和(非)药典试验规范的临床前和临床批次。获得的 SF2a-TT15 候选疫苗通过了所有毒性相关标准,在兔子中具有免疫原性,并在小鼠中引发杀菌抗体。值得注意的是,诱导的 IgG 抗体可识别大量 SF2a 循环菌株。这些临床前数据为 SF2a-TT15 的首次人体研究铺平了道路,证明了安全性和免疫原性。这一贡献揭示了结合疫苗的 GMP 合成尚未报道的可行性,该疫苗具有独特的均质合成聚糖半抗原,经过微调以预防传染病。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号