当前位置:
X-MOL 学术
›
Pharmacol. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Post-Traumatic Epilepsy and Comorbidities: Advanced Models, Molecular Mechanisms, Biomarkers, and Novel Therapeutic Interventions
Pharmacological Reviews ( IF 19.3 ) Pub Date : 2022-04-01 , DOI: 10.1124/pharmrev.121.000375
Victoria M Golub 1 , Doodipala Samba Reddy 2
Pharmacological Reviews ( IF 19.3 ) Pub Date : 2022-04-01 , DOI: 10.1124/pharmrev.121.000375
Victoria M Golub 1 , Doodipala Samba Reddy 2
Affiliation
|
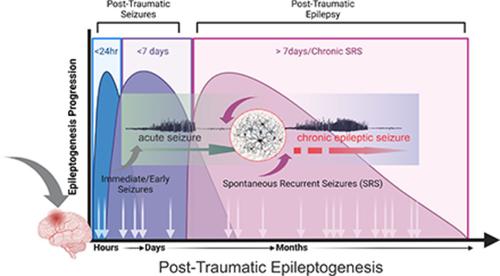
Post-traumatic epilepsy (PTE) is one of the most devastating long-term, network consequences of traumatic brain injury (TBI). There is currently no approved treatment that can prevent onset of spontaneous seizures associated with brain injury, and many cases of PTE are refractory to antiseizure medications. Post-traumatic epileptogenesis is an enduring process by which a normal brain exhibits hypersynchronous excitability after a head injury incident. Understanding the neural networks and molecular pathologies involved in epileptogenesis are key to preventing its development or modifying disease progression. In this article, we describe a critical appraisal of the current state of PTE research with an emphasis on experimental models, molecular mechanisms of post-traumatic epileptogenesis, potential biomarkers, and the burden of PTE-associated comorbidities. The goal of epilepsy research is to identify new therapeutic strategies that can prevent PTE development or interrupt the epileptogenic process and relieve associated neuropsychiatric comorbidities. Therefore, we also describe current preclinical and clinical data on the treatment of PTE sequelae. Differences in injury patterns, latency period, and biomarkers are outlined in the context of animal model validation, pathophysiology, seizure frequency, and behavior. Improving TBI recovery and preventing seizure onset are complex and challenging tasks; however, much progress has been made within this decade demonstrating disease modifying, anti-inflammatory, and neuroprotective strategies, suggesting this goal is pragmatic. Our understanding of PTE is continuously evolving, and improved preclinical models allow for accelerated testing of critically needed novel therapeutic interventions in military and civilian persons at high risk for PTE and its devastating comorbidities.
中文翻译:

创伤后癫痫和合并症:高级模型、分子机制、生物标志物和新的治疗干预
创伤后癫痫 (PTE) 是创伤性脑损伤 (TBI) 最具破坏性的长期网络后果之一。目前没有批准的治疗方法可以预防与脑损伤相关的自发性癫痫发作,并且许多 PTE 病例对抗癫痫药物无效。创伤后癫痫发生是一个持久的过程,正常大脑在头部受伤事件后表现出超同步兴奋性。了解癫痫发生中涉及的神经网络和分子病理学是预防其发展或改变疾病进展的关键。在本文中,我们描述了对 PTE 研究现状的批判性评估,重点是实验模型、创伤后癫痫发生的分子机制、潜在的生物标志物、以及 PTE 相关合并症的负担。癫痫研究的目标是确定可以预防 PTE 发展或中断致癫痫过程并缓解相关神经精神合并症的新治疗策略。因此,我们还描述了当前有关 PTE 后遗症治疗的临床前和临床数据。在动物模型验证、病理生理学、癫痫发作频率和行为的背景下概述了损伤模式、潜伏期和生物标志物的差异。改善 TBI 恢复和预防癫痫发作是复杂且具有挑战性的任务;然而,在这十年中取得了很大进展,证明了疾病改善、抗炎和神经保护策略,表明这一目标是务实的。我们对 PTE 的理解在不断发展,
更新日期:2022-03-17
中文翻译:

创伤后癫痫和合并症:高级模型、分子机制、生物标志物和新的治疗干预
创伤后癫痫 (PTE) 是创伤性脑损伤 (TBI) 最具破坏性的长期网络后果之一。目前没有批准的治疗方法可以预防与脑损伤相关的自发性癫痫发作,并且许多 PTE 病例对抗癫痫药物无效。创伤后癫痫发生是一个持久的过程,正常大脑在头部受伤事件后表现出超同步兴奋性。了解癫痫发生中涉及的神经网络和分子病理学是预防其发展或改变疾病进展的关键。在本文中,我们描述了对 PTE 研究现状的批判性评估,重点是实验模型、创伤后癫痫发生的分子机制、潜在的生物标志物、以及 PTE 相关合并症的负担。癫痫研究的目标是确定可以预防 PTE 发展或中断致癫痫过程并缓解相关神经精神合并症的新治疗策略。因此,我们还描述了当前有关 PTE 后遗症治疗的临床前和临床数据。在动物模型验证、病理生理学、癫痫发作频率和行为的背景下概述了损伤模式、潜伏期和生物标志物的差异。改善 TBI 恢复和预防癫痫发作是复杂且具有挑战性的任务;然而,在这十年中取得了很大进展,证明了疾病改善、抗炎和神经保护策略,表明这一目标是务实的。我们对 PTE 的理解在不断发展,

































 京公网安备 11010802027423号
京公网安备 11010802027423号