当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Two Steps to Bicyclo[4.2.0]octadienes from Cyclooctatetraene: Total Synthesis of Kingianic Acid A
Organic Letters ( IF 4.9 ) Pub Date : 2022-03-16 , DOI: 10.1021/acs.orglett.2c00325
Harshal D Patel 1 , Thomas Fallon 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-03-16 , DOI: 10.1021/acs.orglett.2c00325
Harshal D Patel 1 , Thomas Fallon 1
Affiliation
|
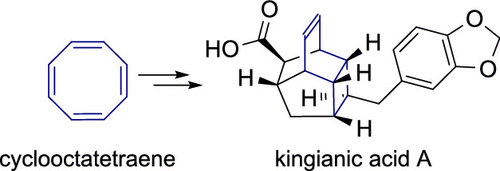
Synthetic approaches to bicyclo[4.2.0]octadiene natural products frequently employ the synthesis of linear tetraenes to initiate a biosynthetic 8π/6π-electrocyclization cascade. This work forges a functionalized bicyclo[4.2.0]octadiene in two steps from cyclooctatetraene. The versatility of this method is demonstrated through natural product synthesis, including the first total synthesis of kingianic acid A and formal syntheses of kingianins A, D, and F and cryptobeilic acid D ethyl ester. The unexpected formation of an E,E,Z,E-tetraene byproduct is rationalized through density functional theory modeling.
中文翻译:

从环辛四烯到双环[4.2.0]辛二烯的两个步骤:金刚酸A的全合成
双环[4.2.0]辛二烯天然产物的合成方法经常使用线性四烯的合成来启动生物合成的8π/6π-电环化级联。这项工作从环辛四烯分两步锻造功能化的双环[4.2.0]辛二烯。该方法的多功能性通过天然产物合成得到证实,包括首次全合成金刚烷酸 A 以及金刚烷素 A、D 和 F 和隐贝酸 D 乙酯的正式合成。通过密度泛函理论建模使E、E、Z、E -四烯副产物的意外形成合理化。
更新日期:2022-03-16
中文翻译:

从环辛四烯到双环[4.2.0]辛二烯的两个步骤:金刚酸A的全合成
双环[4.2.0]辛二烯天然产物的合成方法经常使用线性四烯的合成来启动生物合成的8π/6π-电环化级联。这项工作从环辛四烯分两步锻造功能化的双环[4.2.0]辛二烯。该方法的多功能性通过天然产物合成得到证实,包括首次全合成金刚烷酸 A 以及金刚烷素 A、D 和 F 和隐贝酸 D 乙酯的正式合成。通过密度泛函理论建模使E、E、Z、E -四烯副产物的意外形成合理化。

































 京公网安备 11010802027423号
京公网安备 11010802027423号