当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Radical-mediated C–N bond activation in 3,5-diamino-4-nitro-1H-pyrazole towards high-energy and insensitive energetic materials
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2022-03-16 , DOI: 10.1039/d2ta01146h Zhiwei Zeng 1 , Yuji Liu 1 , Wei Huang 1 , Jean'ne M. Shreeve 2 , Yongxing Tang 1
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2022-03-16 , DOI: 10.1039/d2ta01146h Zhiwei Zeng 1 , Yuji Liu 1 , Wei Huang 1 , Jean'ne M. Shreeve 2 , Yongxing Tang 1
Affiliation
|
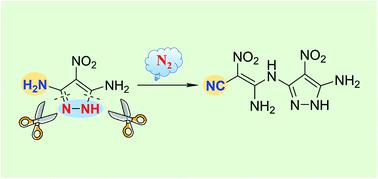
C–N bond activation has been recognized as a powerful methodology in organic synthesis, but C–N bond activation on monocyclic rings remains an intractable challenge, due to the high energy barrier required for the dearomatization process. Now a facile and efficient nitrogen-centered-radical-mediated approach to cleave C–N bonds in a monocyclic pyrazole is described. Using N-bromosuccinimide as a radical initiator, C–N bond cleavage was achieved in yields up to 91%. This reaction led to an important precursor (2), subsequently annulated and oxidized to an energetic compound 4, which exhibits promising application in balancing performances and thermal stabilities.
中文翻译:

3,5-二氨基-4-硝基-1H-吡唑中自由基介导的C-N键活化对高能和不敏感的含能材料
C-N键活化已被公认为有机合成中的一种强有力的方法,但由于脱芳构化过程所需的高能垒,单环上的C-N键活化仍然是一个棘手的挑战。现在描述了一种简便有效的以氮为中心的自由基介导的方法来裂解单环吡唑中的 C-N 键。使用N-溴代琥珀酰亚胺作为自由基引发剂,C-N 键断裂的产率高达 91%。该反应产生了一种重要的前体 ( 2 ),随后环化并氧化为高能化合物4,该化合物在平衡性能和热稳定性方面具有广阔的应用前景。
更新日期:2022-03-16
中文翻译:

3,5-二氨基-4-硝基-1H-吡唑中自由基介导的C-N键活化对高能和不敏感的含能材料
C-N键活化已被公认为有机合成中的一种强有力的方法,但由于脱芳构化过程所需的高能垒,单环上的C-N键活化仍然是一个棘手的挑战。现在描述了一种简便有效的以氮为中心的自由基介导的方法来裂解单环吡唑中的 C-N 键。使用N-溴代琥珀酰亚胺作为自由基引发剂,C-N 键断裂的产率高达 91%。该反应产生了一种重要的前体 ( 2 ),随后环化并氧化为高能化合物4,该化合物在平衡性能和热稳定性方面具有广阔的应用前景。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号