当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rational Design, Synthesis, and Mechanism of (3S,4R)-3-Amino-4-(difluoromethyl)cyclopent-1-ene-1-carboxylic Acid: Employing a Second-Deprotonation Strategy for Selectivity of Human Ornithine Aminotransferase over GABA Aminotransferase
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-03-16 , DOI: 10.1021/jacs.2c00924 Wei Zhu 1 , Arseniy Butrin 2 , Rafael D Melani 3 , Peter F Doubleday 3 , Glaucio Monteiro Ferreira 4 , Mauricio T Tavares 5 , Thahani S Habeeb Mohammad 1 , Brett A Beaupre 2 , Neil L Kelleher 1, 3 , Graham R Moran 2 , Dali Liu 2 , Richard B Silverman 1, 3, 6
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-03-16 , DOI: 10.1021/jacs.2c00924 Wei Zhu 1 , Arseniy Butrin 2 , Rafael D Melani 3 , Peter F Doubleday 3 , Glaucio Monteiro Ferreira 4 , Mauricio T Tavares 5 , Thahani S Habeeb Mohammad 1 , Brett A Beaupre 2 , Neil L Kelleher 1, 3 , Graham R Moran 2 , Dali Liu 2 , Richard B Silverman 1, 3, 6
Affiliation
|
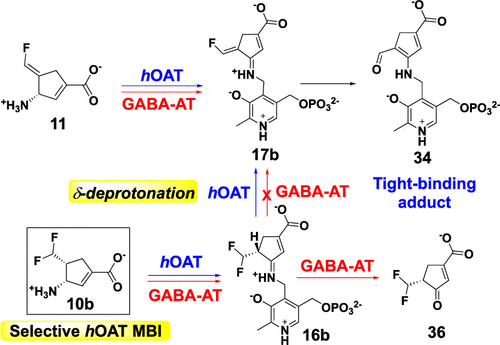
Human ornithine aminotransferase (hOAT) is a pyridoxal 5′-phosphate (PLP)-dependent enzyme that contains a similar active site to that of γ-aminobutyric acid aminotransferase (GABA-AT). Recently, pharmacological inhibition of hOAT was recognized as a potential therapeutic approach for hepatocellular carcinoma. In this work, we first studied the inactivation mechanisms of hOAT by two well-known GABA-AT inactivators (CPP-115 and OV329). Inspired by the inactivation mechanistic difference between these two aminotransferases, a series of analogues were designed and synthesized, leading to the discovery of analogue 10b as a highly selective and potent hOAT inhibitor. Intact protein mass spectrometry, protein crystallography, and dialysis experiments indicated that 10b was converted to an irreversible tight-binding adduct (34) in the active site of hOAT, as was the unsaturated analogue (11). The comparison of kinetic studies between 10b and 11 suggested that the active intermediate (17b) was only generated in hOAT and not in GABA-AT. Molecular docking studies and pKa computational calculations highlighted the importance of chirality and the endocyclic double bond for inhibitory activity. The turnover mechanism of 10b was supported by mass spectrometric analysis of dissociable products and fluoride ion release experiments. Notably, the stopped-flow experiments were highly consistent with the proposed mechanism, suggesting a relatively slow hydrolysis rate for hOAT. The novel second-deprotonation mechanism of 10b contributes to its high potency and significantly enhanced selectivity for hOAT inhibition.
中文翻译:

(3S,4R)-3-Amino-4-(difluoromethyl)cyclopent-1-ene-1-carboxylic Acid 的合理设计、合成和机理:采用二次去质子化策略提高人鸟氨酸氨基转移酶对 GABA 氨基转移酶的选择性
人鸟氨酸转氨酶 (hOAT) 是一种 5'-磷酸吡哆醛 (PLP) 依赖性酶,其活性位点与 γ-氨基丁酸转氨酶 (GABA-AT) 相似。最近,hOAT 的药理学抑制被认为是肝细胞癌的潜在治疗方法。在这项工作中,我们首先研究了两种著名的 GABA-AT 灭活剂(CPP-115和OV329)对 hOAT 的灭活机制。受这两种氨基转移酶灭活机制差异的启发,设计并合成了一系列类似物,从而发现了类似物10b作为一种高选择性和有效的 hOAT 抑制剂。完整的蛋白质质谱、蛋白质晶体学和透析实验表明,10b在 hOAT 的活性位点转化为不可逆的紧密结合加合物 ( 34 ),不饱和类似物 ( 11 )也是如此。10b和11之间的动力学研究比较表明,活性中间体 ( 17b ) 仅在 hOAT 中产生,而不在 GABA-AT 中产生。分子对接研究和 p Ka计算计算突出了手性和环内双键对抑制活性的重要性。10b的周转机制得到可分离产物的质谱分析和氟离子释放实验的支持。值得注意的是,停流实验与提出的机制高度一致,表明 hOAT 的水解速率相对较慢。10b的新型二次去质子化机制有助于提高其效力并显着增强 hOAT 抑制的选择性。
更新日期:2022-03-16
中文翻译:

(3S,4R)-3-Amino-4-(difluoromethyl)cyclopent-1-ene-1-carboxylic Acid 的合理设计、合成和机理:采用二次去质子化策略提高人鸟氨酸氨基转移酶对 GABA 氨基转移酶的选择性
人鸟氨酸转氨酶 (hOAT) 是一种 5'-磷酸吡哆醛 (PLP) 依赖性酶,其活性位点与 γ-氨基丁酸转氨酶 (GABA-AT) 相似。最近,hOAT 的药理学抑制被认为是肝细胞癌的潜在治疗方法。在这项工作中,我们首先研究了两种著名的 GABA-AT 灭活剂(CPP-115和OV329)对 hOAT 的灭活机制。受这两种氨基转移酶灭活机制差异的启发,设计并合成了一系列类似物,从而发现了类似物10b作为一种高选择性和有效的 hOAT 抑制剂。完整的蛋白质质谱、蛋白质晶体学和透析实验表明,10b在 hOAT 的活性位点转化为不可逆的紧密结合加合物 ( 34 ),不饱和类似物 ( 11 )也是如此。10b和11之间的动力学研究比较表明,活性中间体 ( 17b ) 仅在 hOAT 中产生,而不在 GABA-AT 中产生。分子对接研究和 p Ka计算计算突出了手性和环内双键对抑制活性的重要性。10b的周转机制得到可分离产物的质谱分析和氟离子释放实验的支持。值得注意的是,停流实验与提出的机制高度一致,表明 hOAT 的水解速率相对较慢。10b的新型二次去质子化机制有助于提高其效力并显着增强 hOAT 抑制的选择性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号