当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Manganese-Catalyzed Reformation of Vicinal Glycols to α-Hydroxy Carboxylic Acids with the Liberation of Hydrogen Gas
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-03-15 , DOI: 10.1021/acscatal.1c05844 Satyadeep Waiba 1 , Mamata Maiti 1 , Biplab Maji 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-03-15 , DOI: 10.1021/acscatal.1c05844 Satyadeep Waiba 1 , Mamata Maiti 1 , Biplab Maji 1
Affiliation
|
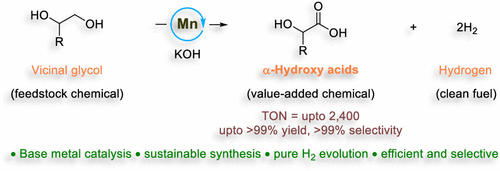
Conversion of readily available feedstocks to valuable platform chemicals via an eco-friendly catalytic pathway has always been one of the key focuses of synthetic chemists. In this context, herein, we report selective transformation of readily available feedstock, vicinal glycols, to value-added α-hydroxycarboxylic acid molecules that are prevalent in bioactive molecules and biodegradable polymers. A bench stable Earth-abundant metal complex, {[HN(C2H4PPh2)2]Mn(CO)2Br}, Mn-I catalyzed the reformation reaction at low temperature in high selectivity with a turnover number reaching 2400, surpassing previously used homogeneous catalysts for such a reaction. Hydrogen gas is evolved as a byproduct without needing an acceptor. The developed protocol is applicable for both aromatic and aliphatic vicinal glycols, delivering the α-substituted hydroxycarboxylic acids in high yields and selectivities. Detailed mechanistic studies elucidated the involvements of different manganese(I)-species during this acceptorless dehydrogenation catalysis.
中文翻译:

释放氢气的锰催化邻二醇重整为α-羟基羧酸
通过环保催化途径将现成的原料转化为有价值的平台化学品一直是合成化学家关注的重点之一。在此背景下,我们报告了将容易获得的原料(邻二醇)选择性转化为在生物活性分子和可生物降解聚合物中普遍存在的增值α-羟基羧酸分子。一种稳定的地球丰富的金属配合物,{[HN(C 2 H 4 PPh 2 ) 2 ]Mn(CO) 2 Br},Mn-I在低温下以高选择性催化重整反应,周转数达到2400,超过了以前用于该反应的均相催化剂。氢气作为副产品释放,不需要受体。开发的协议适用于芳香族和脂肪族邻二醇,以高产率和选择性提供α-取代的羟基羧酸。详细的机理研究阐明了在这种无受体脱氢催化过程中不同锰 (I) 物种的参与。
更新日期:2022-03-15
中文翻译:

释放氢气的锰催化邻二醇重整为α-羟基羧酸
通过环保催化途径将现成的原料转化为有价值的平台化学品一直是合成化学家关注的重点之一。在此背景下,我们报告了将容易获得的原料(邻二醇)选择性转化为在生物活性分子和可生物降解聚合物中普遍存在的增值α-羟基羧酸分子。一种稳定的地球丰富的金属配合物,{[HN(C 2 H 4 PPh 2 ) 2 ]Mn(CO) 2 Br},Mn-I在低温下以高选择性催化重整反应,周转数达到2400,超过了以前用于该反应的均相催化剂。氢气作为副产品释放,不需要受体。开发的协议适用于芳香族和脂肪族邻二醇,以高产率和选择性提供α-取代的羟基羧酸。详细的机理研究阐明了在这种无受体脱氢催化过程中不同锰 (I) 物种的参与。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号