Joule ( IF 38.6 ) Pub Date : 2022-03-14 , DOI: 10.1016/j.joule.2022.02.015
Lang Huang 1 , Tao Lu 1 , Gaojie Xu 1 , Xiaohu Zhang 1 , Zhaoxuan Jiang 1 , Zengqi Zhang 1 , Yantao Wang 1, 2 , Pengxian Han 1 , Guanglei Cui 1, 2 , Liquan Chen 3
|
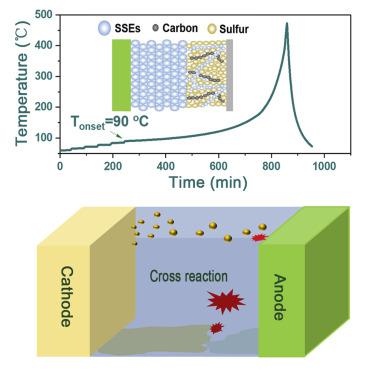
Lithium-sulfur (Li-S) batteries emerge as one of the most attractive energy storage systems due to their ultra-high theoretical energy densities, but the pace of their thermal safety assessment is obviously lagging behind. Herein, by investigating the thermal runaway behavior of Li-S pouch cells from the materials level, we unprecedentedly revealed that the thermal runaway route starts from cathode-induced reactions and then gets accelerated by reactions from the anode. Besides, the solvent vaporization is verified to dominate pressure building up during thermal runaway. Moreover, Li-S batteries employing varied electrolytes with different thermal stabilities, even inorganic all solid-state electrolytes, all undergo rapid thermal runaway at a narrow temperature range due to the intrinsic thermal features of the sulfur cathode and Li metal anode sublimating, melting, and cross-reacting at high temperatures. The in-depth depicted thermal runaway routes will deliver great inspiration for mitigating the safety issues of next generation batteries.
中文翻译:

大规格锂硫软包电池的热失控路线
锂硫(Li-S)电池因其超高的理论能量密度而成为最具吸引力的储能系统之一,但其热安全评估的步伐明显滞后。在此,通过从材料层面研究 Li-S 软包电池的热失控行为,我们前所未有地揭示了热失控路线从阴极诱导反应开始,然后通过阳极反应加速。此外,证实了溶剂蒸发在热失控过程中主导压力的增加。此外,锂硫电池采用具有不同热稳定性的各种电解质,甚至是无机全固态电解质,由于硫正极和锂金属负极在高温下升华、熔化和交叉反应的固有热特性,所有这些都在狭窄的温度范围内经历了快速的热失控。深入描绘的热失控路线将为减轻下一代电池的安全问题提供极大的启发。































 京公网安备 11010802027423号
京公网安备 11010802027423号