当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quinonoid versus Aromatic π-Conjugated Oligomers and Polymers and Their Diradical Characters
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2022-03-14 , DOI: 10.1021/acs.jpcc.2c00113 Girishma Grover 1 , John D. Tovar 2, 3 , Miklos Kertesz 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2022-03-14 , DOI: 10.1021/acs.jpcc.2c00113 Girishma Grover 1 , John D. Tovar 2, 3 , Miklos Kertesz 1
Affiliation
|
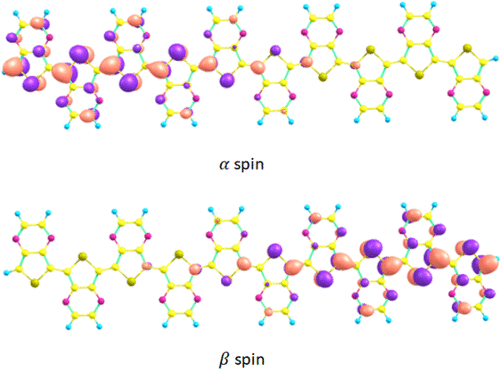
Quinonoid elements in π-conjugated oligomers and polymers have promising characteristics by providing unique structures and electronic properties, including varying degrees of ground state open shell diradical character and small band gaps. So far, quinonoid ground state polymers were rarely identified, and one of the goals of this work was to expand the portfolio of such systems in which over 17 different repeat units were considered, about half preferred a quinonoid and half preferred aromatic structures plus several with the alternating A-Q composition were also included. The dependency of the diradical character as a function of size and composition opens a rich space to discover trends affecting the aromaticity and diradical character. Herein, we show that the diradical character is a useful parameter to classify a wide variety of polymers based on their ground states in a systematic study of a large number of homo- and hetero-systems. Homo-polymers with quinonoid ground states show a high diradical character, while those with the aromatic ground state behave as closed-shell systems with no or a very small diradical character. The diradical character of quinonoid systems depends strongly on the size, which increases with the size of the oligomer. We found a correlation between the diradical character and the interring C–C distance as well as the singlet–triplet energy gaps. In alternating copolymers mixing quinonoid and aromatic subunits, we found open-shell hetero-polymers with moderate to high diradical characters.
中文翻译:

醌类化合物与芳香族 π-共轭低聚物和聚合物及其双自由基特性
π 共轭低聚物和聚合物中的醌类元素通过提供独特的结构和电子特性(包括不同程度的基态开壳双自由基特性和小带隙)而具有有前途的特性。到目前为止,很少发现醌类基态聚合物,这项工作的目标之一是扩大此类系统的组合,其中考虑了超过 17 个不同的重复单元,大约一半优选醌类和一半优选芳族结构以及几个具有交替的 AQ 成分也包括在内。双自由基特性作为大小和成分的函数的依赖性为发现影响芳香性和双自由基特性的趋势开辟了丰富的空间。在此处,我们表明,在对大量同质和异质系统的系统研究中,双自由基特征是根据其基态对各种聚合物进行分类的有用参数。具有醌类基态的均聚物显示出高双自由基特性,而具有芳香基态的均聚物表现为闭壳系统,没有或具有非常小的双自由基特性。醌类系统的双自由基特征很大程度上取决于大小,它随着低聚物的大小而增加。我们发现了双自由基特征与相互间的 C-C 距离以及单重态 - 三重态能隙之间的相关性。在混合醌类和芳香族亚基的交替共聚物中,我们发现了具有中到高双自由基特征的开壳杂聚物。
更新日期:2022-03-14
中文翻译:

醌类化合物与芳香族 π-共轭低聚物和聚合物及其双自由基特性
π 共轭低聚物和聚合物中的醌类元素通过提供独特的结构和电子特性(包括不同程度的基态开壳双自由基特性和小带隙)而具有有前途的特性。到目前为止,很少发现醌类基态聚合物,这项工作的目标之一是扩大此类系统的组合,其中考虑了超过 17 个不同的重复单元,大约一半优选醌类和一半优选芳族结构以及几个具有交替的 AQ 成分也包括在内。双自由基特性作为大小和成分的函数的依赖性为发现影响芳香性和双自由基特性的趋势开辟了丰富的空间。在此处,我们表明,在对大量同质和异质系统的系统研究中,双自由基特征是根据其基态对各种聚合物进行分类的有用参数。具有醌类基态的均聚物显示出高双自由基特性,而具有芳香基态的均聚物表现为闭壳系统,没有或具有非常小的双自由基特性。醌类系统的双自由基特征很大程度上取决于大小,它随着低聚物的大小而增加。我们发现了双自由基特征与相互间的 C-C 距离以及单重态 - 三重态能隙之间的相关性。在混合醌类和芳香族亚基的交替共聚物中,我们发现了具有中到高双自由基特征的开壳杂聚物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号