当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nitrogen-Centered Radicals in Functionalization of sp2 Systems: Generation, Reactivity, and Applications in Synthesis
Chemical Reviews ( IF 51.4 ) Pub Date : 2022-03-14 , DOI: 10.1021/acs.chemrev.1c00831 Cassie Pratley 1, 2 , Sabine Fenner 2 , John A Murphy 1
Chemical Reviews ( IF 51.4 ) Pub Date : 2022-03-14 , DOI: 10.1021/acs.chemrev.1c00831 Cassie Pratley 1, 2 , Sabine Fenner 2 , John A Murphy 1
Affiliation
|
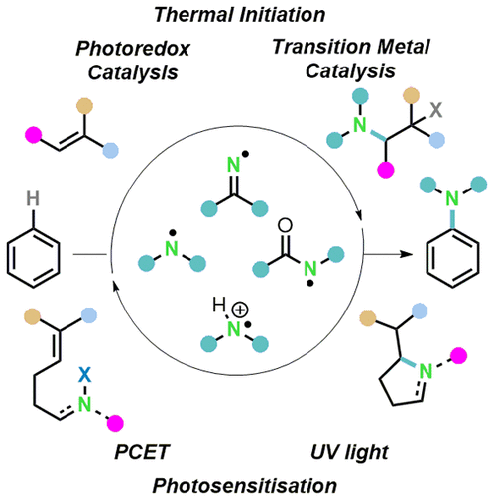
The chemistry of nitrogen-centered radicals (NCRs) has plentiful applications in organic synthesis, and they continue to expand as our understanding of these reactive species increases. The utility of these reactive intermediates is demonstrated in the recent advances in C–H amination and the (di)amination of alkenes. Synthesis of previously challenging structures can be achieved by efficient functionalization of sp2 moieties without prefunctionalization, allowing for faster and more streamlined synthesis. This Review addresses the generation, reactivity, and application of NCRs, including, but not limited to, iminyl, aminyl, amidyl, and aminium species. Contributions from early discovery up to the most recent examples have been highlighted, covering radical initiation, thermolysis, photolysis, and, more recently, photoredox catalysis. Radical-mediated intermolecular amination of (hetero)arenes can occur with a variety of complex amine precursors, generating aniline derivatives, an important class of structures for drug discovery and development. Functionalization of olefins is achievable in high anti-Markovnikov regioselectivity and allows access to difunctionalized structures when the intermediate carbon radicals are trapped. Additionally, the reactivity of NCRs can be harnessed for the rapid construction of N-heterocycles such as pyrrolidines, phenanthridines, quinoxalines, and quinazolinones.
中文翻译:

sp2 系统功能化中的氮中心自由基:生成、反应性和合成中的应用
以氮为中心的自由基 (NCR) 的化学在有机合成中有着广泛的应用,并且随着我们对这些活性物质的了解增加,它们继续扩展。这些反应性中间体的效用在 C-H 胺化和烯烃的(二)胺化的最新进展中得到了证明。可以通过 sp 2的有效功能化来合成以前具有挑战性的结构没有预功能化的部分,允许更快和更流线化的合成。本综述讨论了 NCR 的产生、反应性和应用,包括但不限于亚胺基、胺基、酰胺基和胺类物质。重点介绍了从早期发现到最新例子的贡献,包括自由基引发、热解、光解,以及最近的光氧化还原催化。(杂)芳烃的自由基介导的分子间胺化可以与多种复杂的胺前体发生,产生苯胺衍生物,这是药物发现和开发的重要结构类别。烯烃的功能化可以在高反马尔科夫尼科夫区域选择性中实现,并且当中间碳自由基被捕获时允许访问双功能化结构。此外,
更新日期:2022-03-14
中文翻译:

sp2 系统功能化中的氮中心自由基:生成、反应性和合成中的应用
以氮为中心的自由基 (NCR) 的化学在有机合成中有着广泛的应用,并且随着我们对这些活性物质的了解增加,它们继续扩展。这些反应性中间体的效用在 C-H 胺化和烯烃的(二)胺化的最新进展中得到了证明。可以通过 sp 2的有效功能化来合成以前具有挑战性的结构没有预功能化的部分,允许更快和更流线化的合成。本综述讨论了 NCR 的产生、反应性和应用,包括但不限于亚胺基、胺基、酰胺基和胺类物质。重点介绍了从早期发现到最新例子的贡献,包括自由基引发、热解、光解,以及最近的光氧化还原催化。(杂)芳烃的自由基介导的分子间胺化可以与多种复杂的胺前体发生,产生苯胺衍生物,这是药物发现和开发的重要结构类别。烯烃的功能化可以在高反马尔科夫尼科夫区域选择性中实现,并且当中间碳自由基被捕获时允许访问双功能化结构。此外,































 京公网安备 11010802027423号
京公网安备 11010802027423号